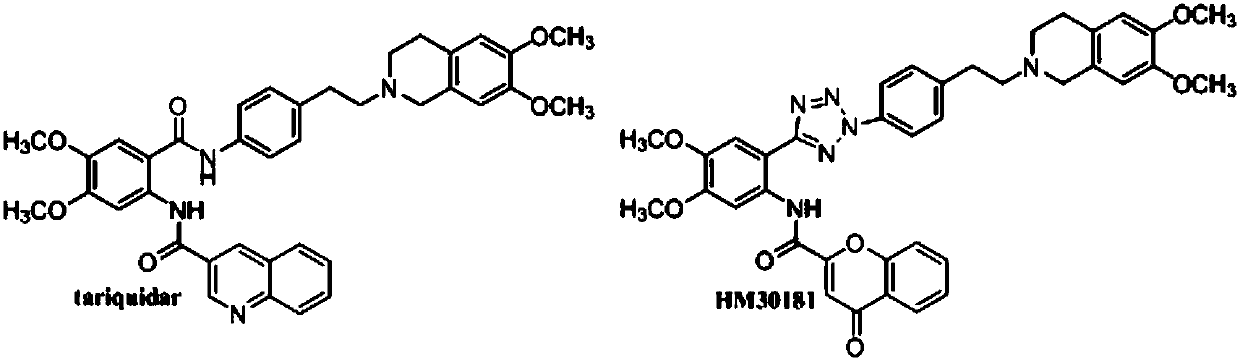
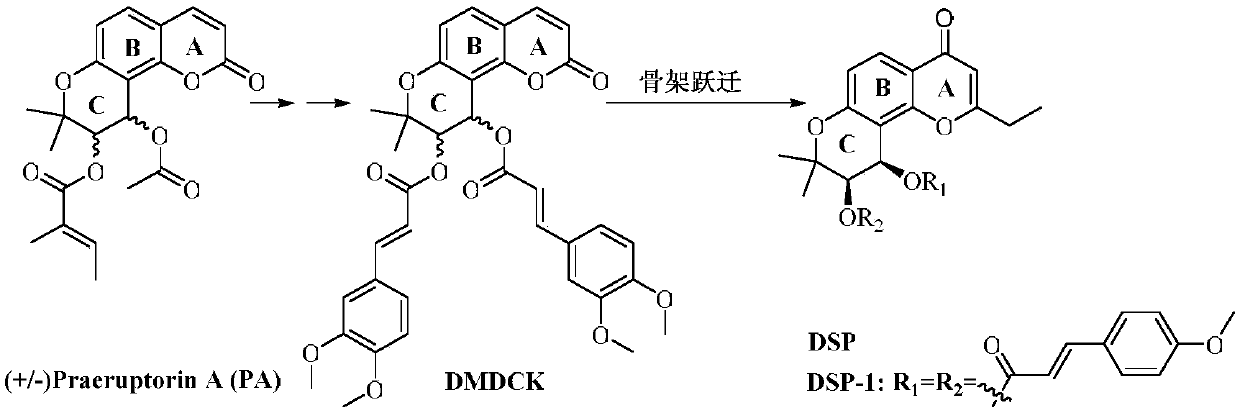
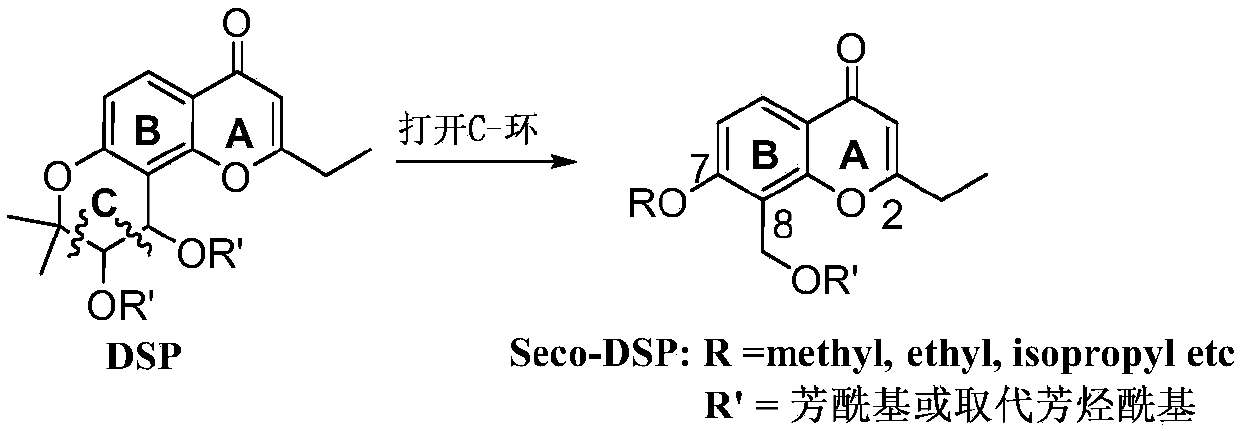
Benzopyrone skeleton derivative, preparation method and uses thereof
A technology of benzopyrone and derivatives, which is applied in the field of chemical pharmacy, can solve the problems of reducing bile discharge, toxicity, and poor selectivity, and achieve the effects of increasing sensitivity, reversing drug resistance, and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of compound 1-(2-hydroxy-4-methoxymethoxy)-phenyl-1,3-pentanedione (1) in general formula 1
[0040] In a three-necked flask, add 60% sodium hydride (8.86g, 221.5mmol), anhydrous tetrahydrofuran (62.4mL), 2-hydroxy-4-methoxymethoxy-acetophenone (13.28mL) dissolved in tetrahydrofuran (55.8mL) g, 67.69mmol), ethyl propionate (104.5mL, 135.37mmol). Reflux for 2 hours, cool to room temperature, adjust the pH to neutral with hydrochloric acid, evaporate most of the tetrahydrofuran, add ice water, extract with dichloromethane several times, combine the organic phases, wash once with water, once with saturated sodium chloride solution, anhydrous Na2SO4 dried. After concentration and drying, the crude compound 1 (16.22 g) was obtained, the crude yield was 95.0%, and it was a light yellow solid, which could be directly carried out to the next reaction without purification.
Embodiment 2
[0042] Synthesis of Compound 2-Ethyl-7-Hydroxy-4H-Chromene-4-one (2) in General Formula 1
[0043] Compound 1 (16.22g, 64.31mmol) was dissolved in ethanol (500mL), concentrated hydrochloric acid (10mL) was added, refluxed for 0.5h, and the crude product 2 was obtained after evaporation of ethanol and drying. The yield was 100%, and it could be carried out directly without purification Next reaction.
Embodiment 3
[0045] Synthesis of Compound 2-Ethyl-7-Hydroxy-8-Formaldehyde-4H-Chromene-4-one (3) in General Formula 1
[0046] Compound 2 (30.0g, 157.7mmol) was dissolved in glacial acetic acid (500mL), added urotropine (154.78g, 1.10mol), heated to 90-100°C for 3h, part of the glacial acetic acid was evaporated; added 5N hydrochloric acid (800mL ), reflux continued to react for 0.5h, the reaction solution was poured into ice water (1000mL) in small streams, a yellow solid was precipitated, filtered, washed with ice water several times until no pungent smell, and after drying, yellow solid compound 3 (8.65g) was obtained , yield 25.13%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com