Use of tryptophan metabolites for treating muscle atrophy
A metabolite, tryptophan technology, applied in the field of tryptophan metabolites, can solve the problem of ineffective protein synthesis, and achieve the effect of improving recovery time and increasing muscle mass
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
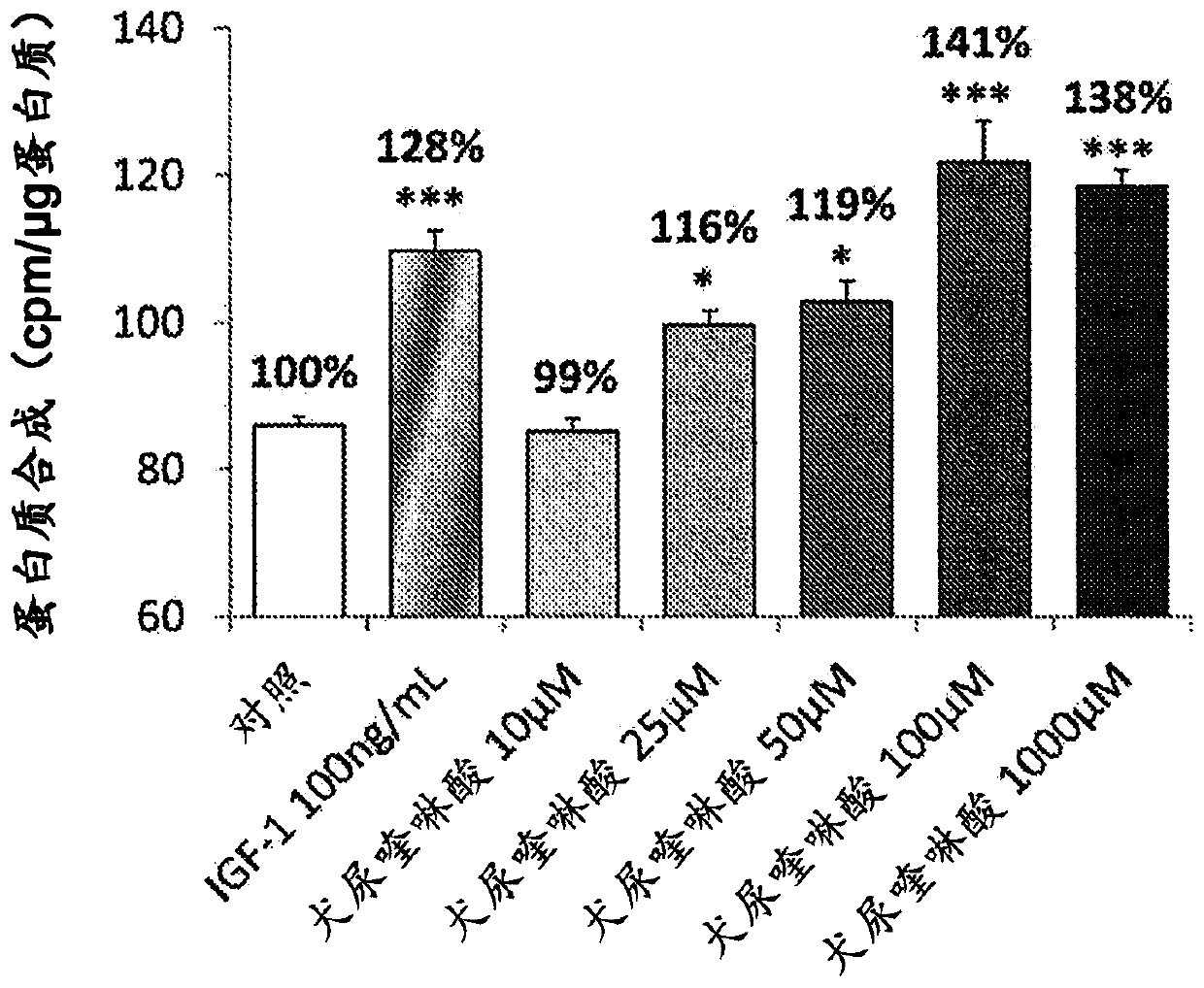
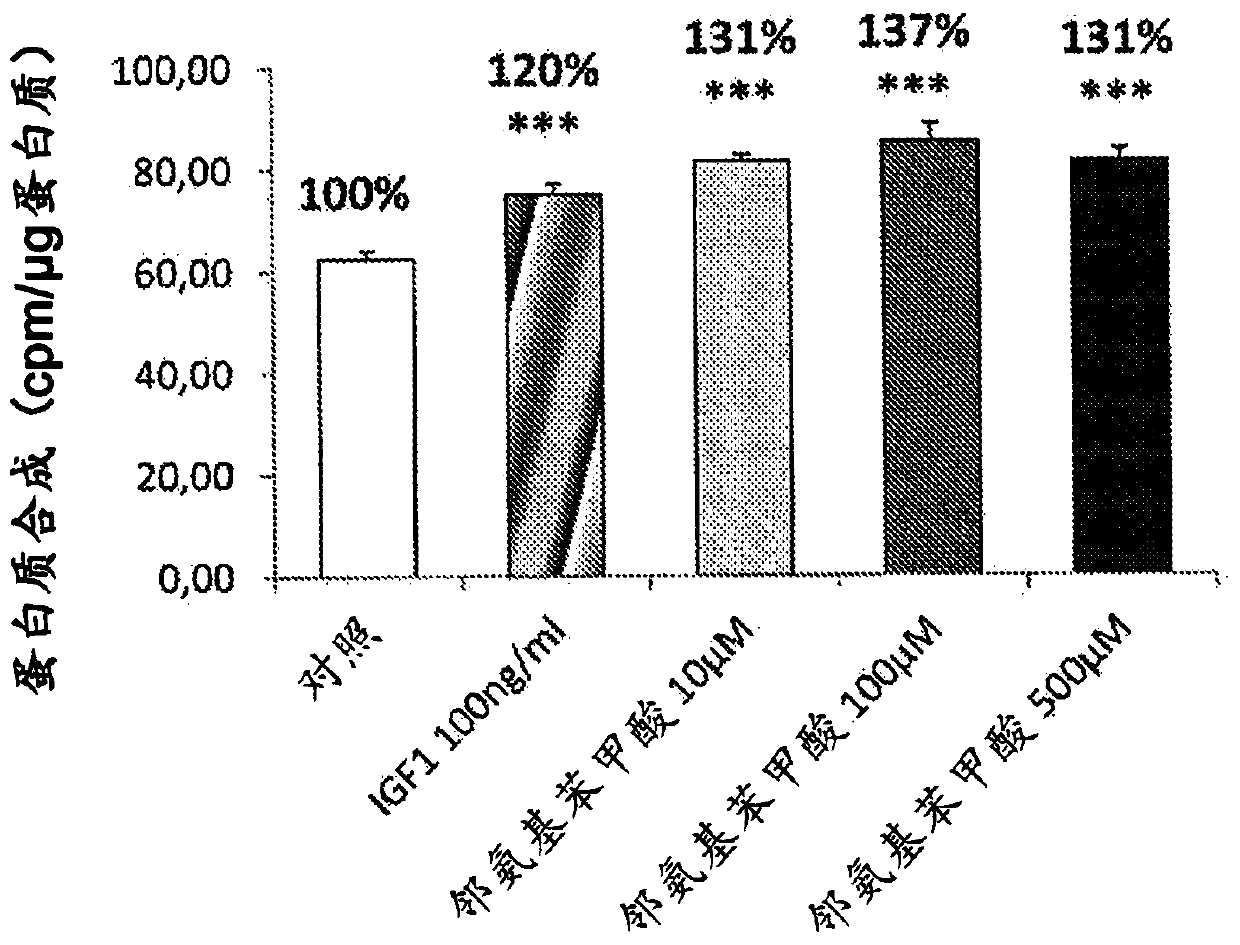
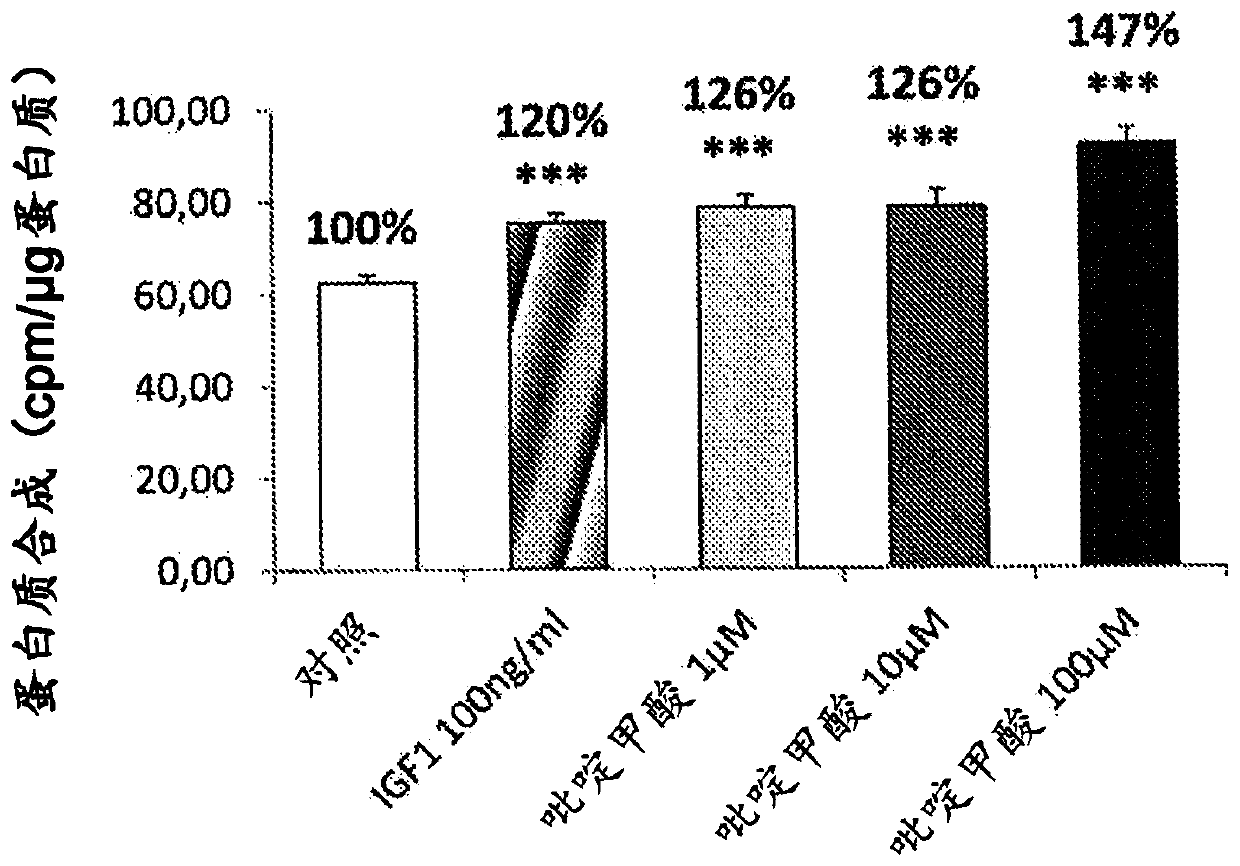
[0067] Example 1: Measurement of protein synthesis in C2C12 cells
[0068] Cells were counted and seeded at a density of 20 000 cells per well in 24-well plates in DMEM supplemented with fetal calf serum (10%) and antibiotics (penicillin and streptomycin) containing glucose at a ratio of 4.5 g / l medium. After 48 hours, myoblast differentiation was induced for 5 days by partial serum deprivation (2% instead of 10%). The cells were then placed in medium without glucose or leucine (Krebs medium) at 37°C for 1 h, and then in serum-free DMEM medium containing 2.5 μCi / ml radiolabeled leucine, at In the presence of the product to be tested (DMSO (control), kynurenic acid or anthranilic acid or quinolinic acid or picolinic acid or 3OH-kynurenine) or reference (IGF-1, 100 ng / ml) Warm bath for 150min. At the end of the incubation, the supernatant was removed and the cells were lysed in 0.1 N NaOH solution for 30 min. Radioactivity was measured in the cellular fraction and the total ...
Embodiment 2
[0069] Example 2: Measurement of Myostatin Gene Expression in C2C12 Cells
[0070] C2C12 myoblasts (ATCC CRL-1772) were seeded in 24-well plates at a density of 30 000 cells per well in a cultured medium containing glucose at a ratio of 4.5 g / l supplemented with fetal calf serum (10%) and antibiotics cultured in DMEM medium (penicillin and streptomycin). After 48 hours, the myoblasts were induced to differentiate for 5 days by partial serum deprivation (2% instead of 10%). Cells were then placed in serum-free, glucose-depleted medium (DMEM containing 1 g / l glucose) in the presence of the molecule to be tested (DMSO (control) or IGF-1 (100 ng / ml) or kynurenic acid ) or reference (IGF-1 at a concentration of 100ng / ml) for 6h. At the end of the experiment, messenger RNA (mRNA) was extracted using conventional methods based on phenol and chloroform. Briefly, cells were lysed in trizol solution (Sigma T9424) containing strong acid and phenol. mRNA was separated from protein by ...
Embodiment 3
[0075] Example 3: Evaluation of C2C12 muscle fiber diameter
[0076]C2C12 myoblasts (ATCC CRL-1772) were seeded at a density of 10 000 cells per well in glycerol-treated 8-well plates in the presence of glucose at a ratio of 4.5 g / l supplemented with fetal bovine serum (10 %) and antibiotics (penicillin and streptomycin) in DMEM medium. After 48 hours, the myoblasts were induced to differentiate for 3 days by partial serum deprivation (2% instead of 10%). Cells were then placed in serum-free, glucose-depleted medium (DMEM containing 1 g / l glucose) in the presence of the molecule to be tested (DMSO (control) or kynurenic acid) or reference (at a concentration of 100 ng / l ml of IGF-1 or 10 μM dexamethasone) for 3 days. At the end of the incubation, cells were washed and fixed using a 2.5% glutaraldehyde / 0.1% triton solution for 1 h at ambient temperature. The cell layer was overlaid with DAPI, a fluorescent marker of the nucleus. After being stored in a dark place for 16 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com