Preparation method of manganese doped double-shell-layer calcium carbonate hollow microsphere CO2 adsorbent
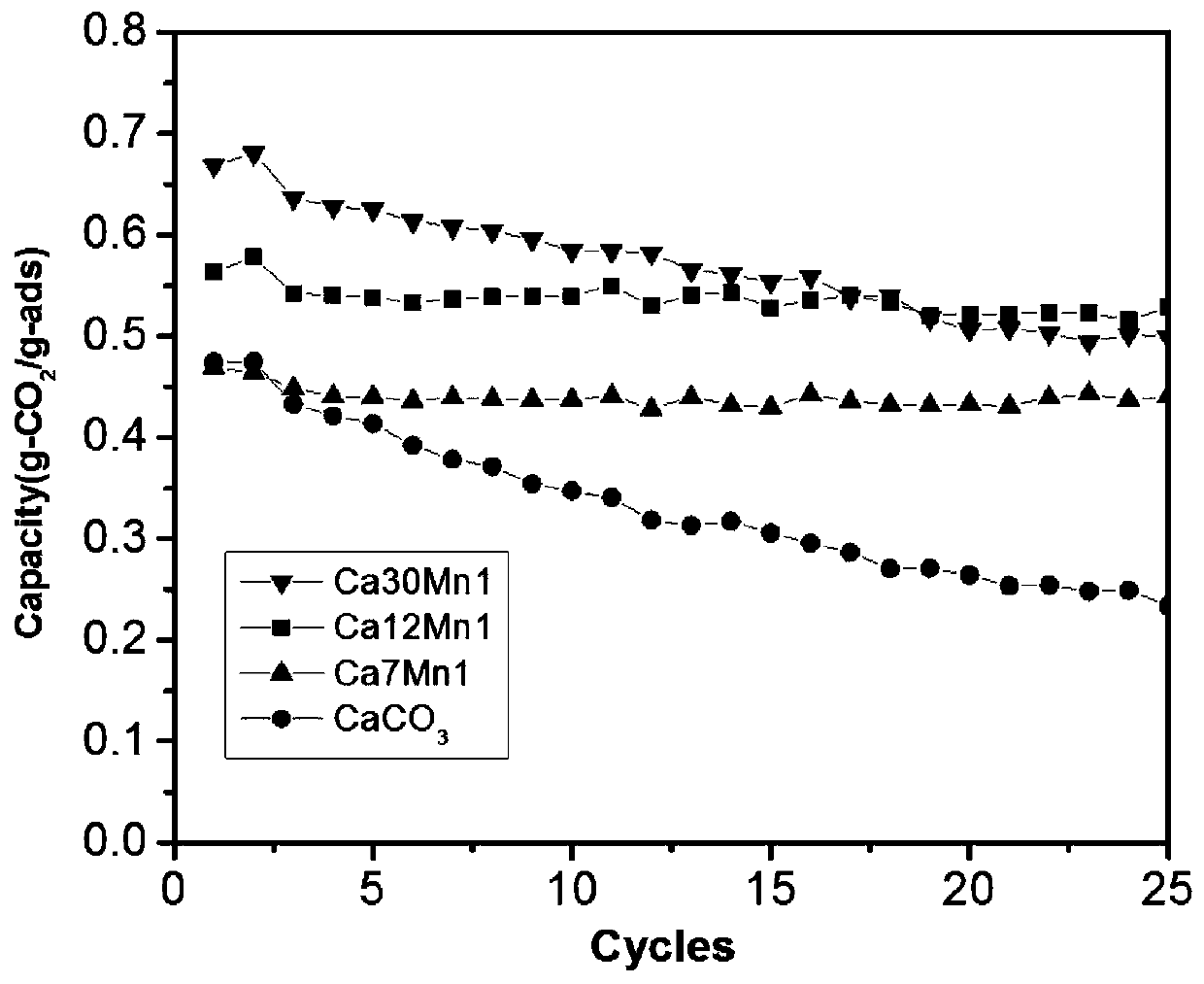
A double-shell, calcium carbonate technology, applied in separation methods, chemical instruments and methods, alkali metal oxides/hydroxides, etc., can solve the problems of calcium-based adsorbents such as easy sintering and rapid deactivation, and achieve excellent adsorption Performance stability, improved adsorption performance, and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
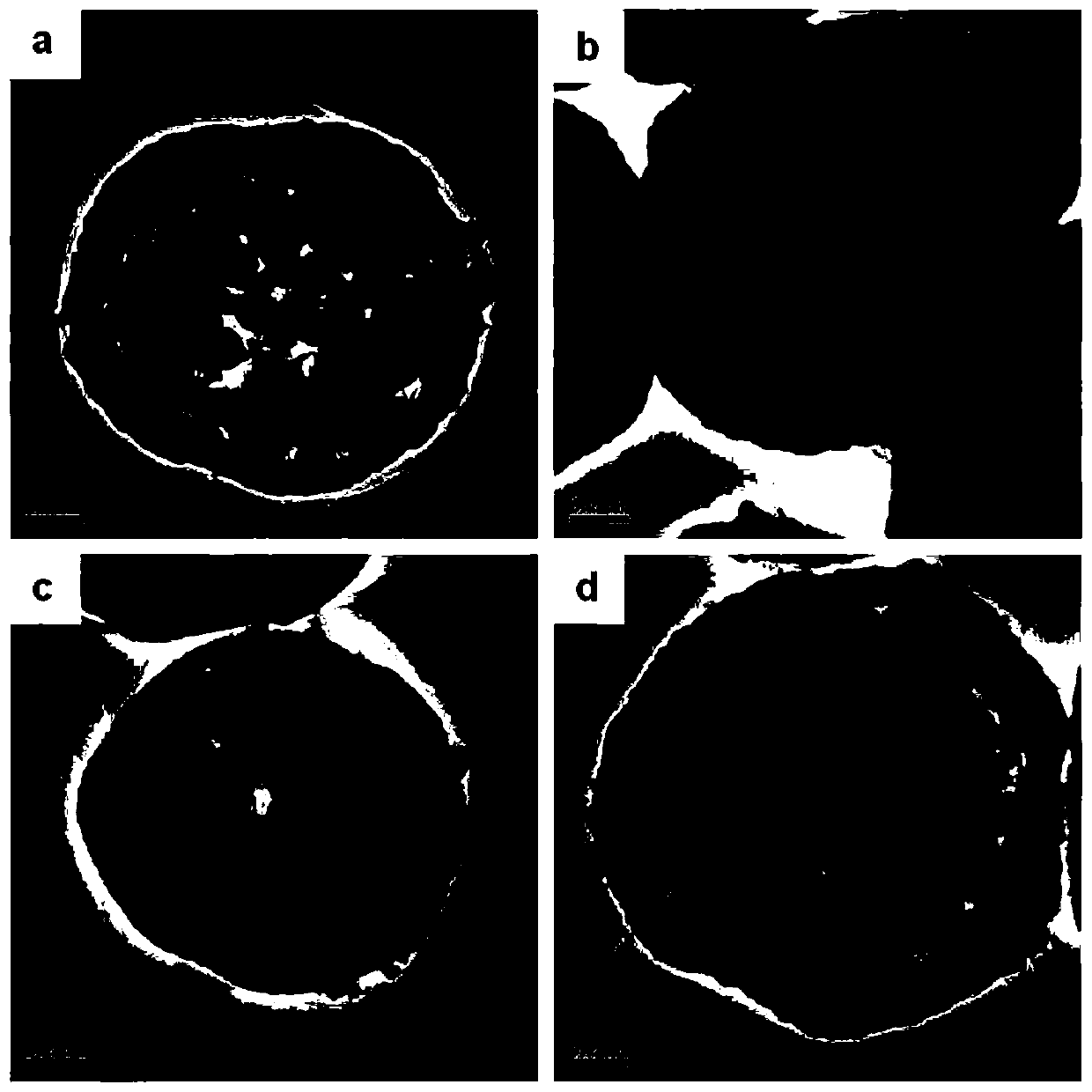
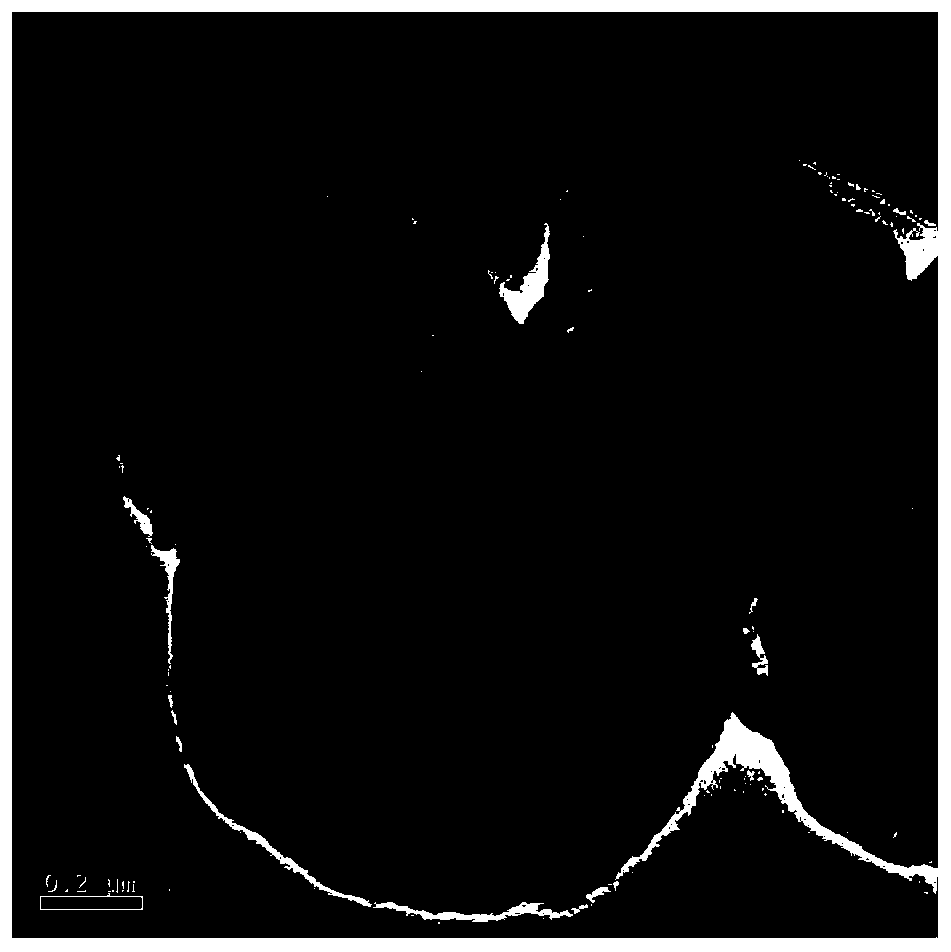
[0028] (1) Preparation of carbon sphere template: put the aqueous solution of sucrose with a concentration of 1M into the crystallization kettle, carry out the hydrothermal crystallization reaction at 200°C for 120min, and suction filter the obtained product after the crystallization is completed. The obtained filter cake was alternately washed with ethanol and water, and the obtained product was dried at 80° C. for 12 hours, and then ground, and the obtained carbon spheres had a diameter of 2 μm.
[0029] (2) Get 20ml of absolute ethanol and 10ml of deionized water to obtain a mixed solvent, and 14.169g of Ca(NO 3 ) 2 4H 2 O and 0.588g of Mn(CH 3 COO) 2 4H 2 O (that is, the concentration of calcium ions is 2mol / L, the molar ratio of calcium and manganese ions fed into the feed is 25 / 1, and finally the molar ratio of calcium and manganese ions actually measured in the adsorbent is 12 / 1) is added to the above solvent to obtain a metal salt solution A. Add 600 mg of the car...
Embodiment 2
[0032] (1) Preparation of carbon sphere template: put the aqueous solution of sucrose with a concentration of 1M into the crystallization kettle, carry out the hydrothermal crystallization reaction at 200°C for 110min, and suction filter the obtained product after the crystallization is completed, and filter the obtained filter cake Alternately washing with ethanol and water several times, followed by drying at 80 °C for 12 h, and grinding, the obtained carbon spheres have a diameter of 1 μm.
[0033] (2) Get 20ml of absolute ethanol and 10ml of deionized water to obtain a mixed solvent, and 14.169g of Ca(NO 3 ) 2 4H 2 O and 0.588g of Mn(CH 3 COO) 2 4H 2 O (that is, the concentration of calcium ions is 2mol / L, the molar ratio of calcium and manganese ions fed into the feed is 25 / 1, and finally the molar ratio of calcium and manganese ions actually measured in the adsorbent is 12 / 1) is added to the above solvent to obtain a metal salt solution A. Add 600 mg of the carbon s...
Embodiment 3
[0036] (1) Preparation of carbon sphere template: put an aqueous solution of sucrose with a concentration of 1M into a crystallization kettle, carry out a hydrothermal crystallization reaction at 200°C for 120min, and suction filter the obtained product after the crystallization is completed, and filter the obtained filter cake Alternately washing with ethanol and water for several times, followed by drying at 80 °C for 12 h, and grinding, the obtained carbon spheres have a diameter of 2 μm.
[0037] (2) Get 30ml of deionized water to obtain a mixed solvent, and 14.169g of Ca(NO 3 ) 2 4H 2 O and 0.588g of Mn(CH 3 COO) 2 4H 2 O (that is, the concentration of calcium ions is 2mol / L, the molar ratio of calcium and manganese ions fed into the feed is 25 / 1, and finally the molar ratio of calcium and manganese ions actually measured in the adsorbent is 12 / 1) is added to the above solvent to obtain a metal salt solution A. Add 600 mg of the carbon sphere template prepared in 1) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com