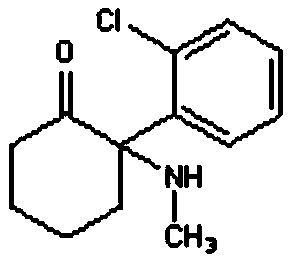
Ketamine transdermal patch and preparation method thereof
A transdermal patch, ketamine technology, applied in anesthetics, anti-inflammatory agents, pharmaceutical formulations, etc., can solve the problem of difficulty in controlling the amount of skin penetration enhancers and crystallization inhibitors, difficulty in completely avoiding ketamine crystallization, and low transdermal penetration rate and other problems, to achieve the effects of easy preparation and use, increased percutaneous penetration rate, and reduced risk of addiction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method of ketamine transdermal patch, comprising the following preparation steps:
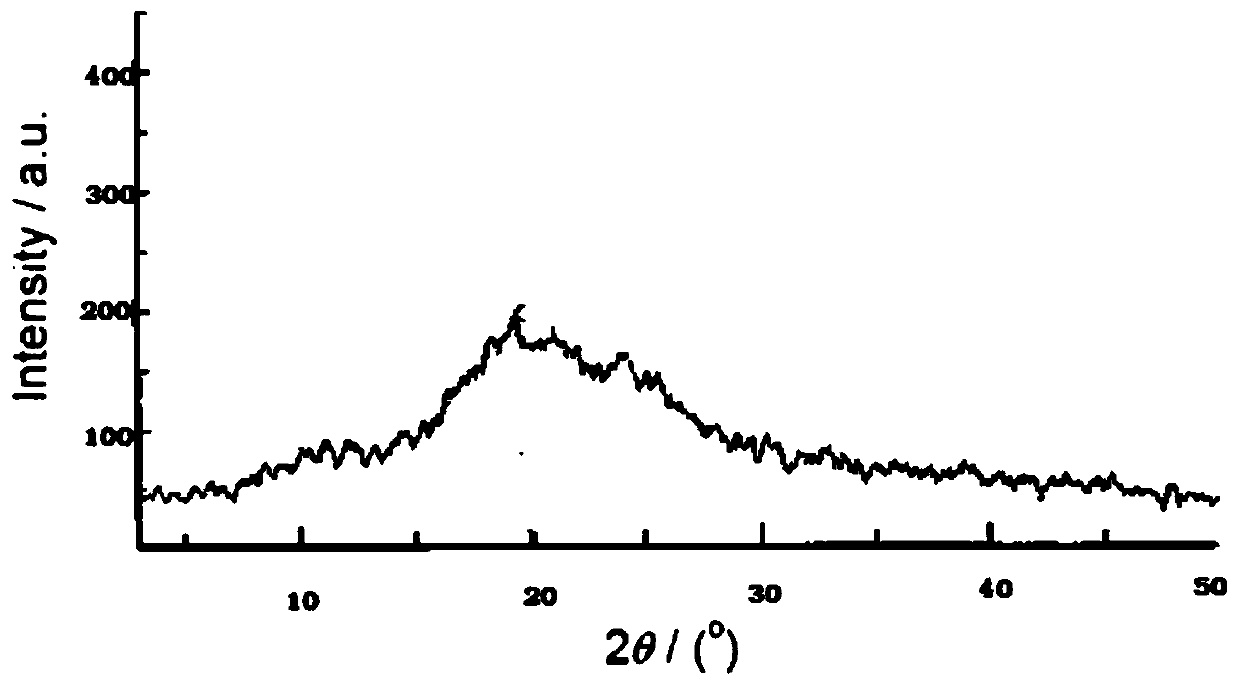
[0038] (1) Decrystallization of ketamine to obtain decrystallization powder, including:
[0039] (1.1) Dissolve 10g of ketamine in 20g of hot ethanol;
[0040] (1.2) Add organic amine (diethanolamine) 30g and soybean lecithin 5g in proportion;
[0041] (1.3) After stirring uniformly at room temperature for 3 hours, spray dry to get it; spray drying conditions: spray dryer; nitrogen flow pressure: 600L / h; air inlet temperature: 90°C; air outlet temperature: 43°C; feed amount: 5mL / min; Needle pass frequency: 15 seconds / time;
[0042] (2) Disperse the decrystallized powder of step (1) in cholesterol and fatty acid to form dispersed microspheres of multiple microreservoirs; including: 77 mg of stearic acid mixed with 7 mL of heptane, 40 mg of cholesterol Heat to 60°C until a clear solution is obtained. and allow to cool to room temperature. Next, a decrystallized powder co...
Embodiment 2
[0046] A preparation method of ketamine transdermal patch, comprising the following preparation steps:
[0047] (1) Decrystallization of ketamine to obtain decrystallization powder, including:
[0048] (1.1) Dissolve 10g of ketamine in 30g of hot ethanol;
[0049] (1.2) Add organic amine (diethanolamine) 20g and soybean lecithin 5g in proportion;
[0050] (1.3) After stirring uniformly at room temperature for 4 hours, spray dry to get it; spray drying conditions: spray dryer; nitrogen flow pressure: 600L / h; air inlet temperature: 90°C; air outlet temperature: 43°C; feed amount: 5mL / min; Needle pass frequency: 15 seconds / time;
[0051] (2) Disperse the decrystallized powder of step (1) in cholesterol and fatty acid to form dispersed microspheres of multiple micro-reservoirs; including: 40 mg of stearic acid mixed with 7 mL of heptane, 40 mg of cholesterol Heat to 60°C until a clear solution is obtained. and allow to cool to room temperature. Next, a decrystallized powder c...
Embodiment 3
[0055] A preparation method of ketamine transdermal patch, comprising the following preparation steps:
[0056] (1) Decrystallization of ketamine to obtain decrystallization powder, including:
[0057] (1.1) Dissolve 10g of ketamine in 20g of hot ethanol;
[0058] (1.2) Add organic amine (triethanolamine) 30g and soybean lecithin 5g in proportion;
[0059] (1.3) After stirring uniformly at room temperature for 3 hours, spray dry to get it; spray drying conditions: spray dryer; nitrogen flow pressure: 600L / h; air inlet temperature: 90°C; air outlet temperature: 43°C; feed amount: 5mL / min; Needle pass frequency: 15 seconds / time;
[0060] (2) Disperse the decrystallized powder of step (1) in cholesterol and fatty acid to form dispersed microspheres of multiple microreservoirs; including: 77 mg of stearic acid mixed with 7 mL of heptane, 40 mg of cholesterol Heat to 60°C until a clear solution is obtained. and allow to cool to room temperature. Next, a decrystallized powder con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com