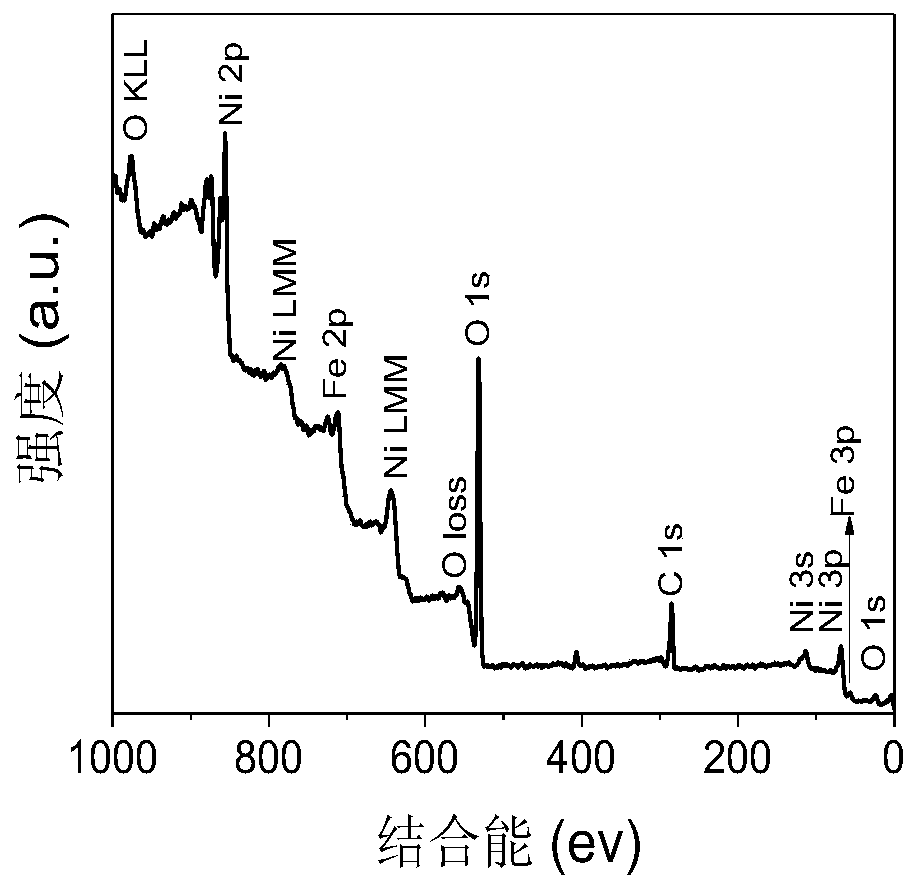
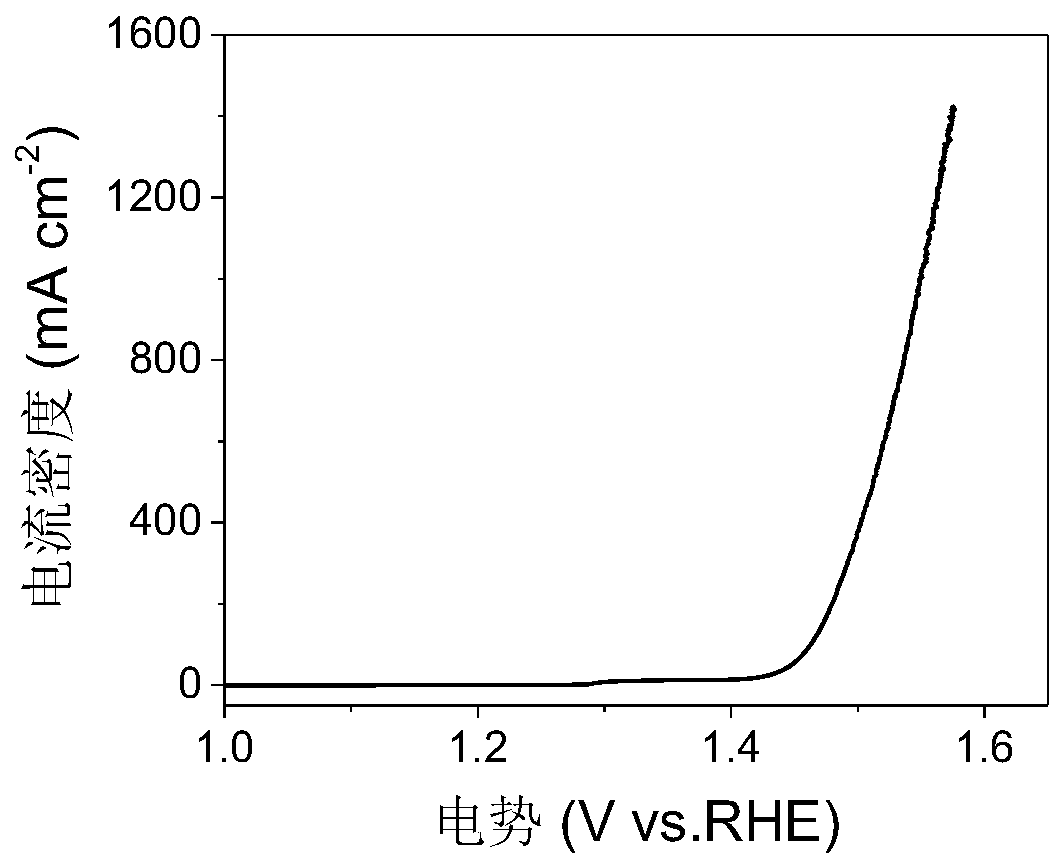
Preparation method and application of amorphous (Ni,Fe)OOH thin film electrocatalyst supported on surface of foamed nickel
A technology of electrocatalyst and nickel foam, which is applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve complex precursors, harsh reaction conditions and other problems, and achieve high-efficiency catalytic activity and effective charge Effect of transfer, high-efficiency electrocatalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Preparation of Ferric Nitrate Solution
[0033] (1) Weigh 3.096g of ferric nitrate nonahydrate, dissolve it in 8mL of distilled water, stir at room temperature until completely dissolved, and the prepared ferric nitrate solution is 1.6mol / L.
[0034] (2) Use a 1000 μL pipette to pipette 1 mL of 1.6 mol / L ferric nitrate solution, and then pipette 7 mL of distilled water for dilution to obtain a 0.2 mol / L ferric nitrate solution.
[0035] 2. Preparation of Foamed Nickel
[0036] (1) Soak nickel foam in 0.1mol / L hydrochloric acid solution, acetone solution, and absolute ethanol solution for 10 minutes, and finally wash it three times with distilled water.
[0037] 3. Preparation of 1.0M KOH solution
[0038] (1) Measure about 50mL of ultrapure water and 5.61g of potassium hydroxide, dissolve them with ultrapure water and stir, after cooling, dilute to volume in a 100mL volumetric flask.
[0039] 4. Preparation of amorphous (Ni,Fe)OOH films supported on the surface of...
Embodiment 2
[0045] 1. Preparation of Ferric Nitrate Solution
[0046] (1) Weigh 3.096g of ferric nitrate nonahydrate, dissolve it in 8mL of distilled water, stir at room temperature until completely dissolved, and the prepared ferric nitrate solution is 1.6mol / L.
[0047] (2) Use a 1000 μL pipette to pipette 1 mL of 1.6 mol / L ferric nitrate solution, and then pipette 15 mL of distilled water for dilution to obtain a 0.1 mol / L ferric nitrate solution.
[0048] 2. Preparation of Foamed Nickel
[0049] (1) Soak nickel foam in 0.1mol / L hydrochloric acid solution, acetone solution, and absolute ethanol solution for 10 minutes, and finally wash it three times with distilled water.
[0050] 3. Preparation of 1.0M KOH solution
[0051] (1) Measure about 50mL of ultrapure water and 5.61g of potassium hydroxide, dissolve them with ultrapure water and stir, after cooling, dilute to volume in a 100mL volumetric flask.
[0052] 4. Preparation of amorphous (Ni,Fe)OOH films supported on the surface of ...
Embodiment 3
[0057] 1. Preparation of Ferric Nitrate Solution
[0058] (1) Weigh 3.096g of ferric nitrate nonahydrate, dissolve it in 8mL of distilled water, stir at room temperature until completely dissolved, and the prepared ferric nitrate solution is 1.6mol / L.
[0059] (2) Use a 1000 μL pipette to pipette 1 mL of 1.6 mol / L ferric nitrate solution, and then pipette 3 mL of distilled water for dilution to obtain a 0.4 mol / L ferric nitrate solution.
[0060] 2. Preparation of Foamed Nickel
[0061] (1) Soak nickel foam in 0.1mol / L hydrochloric acid solution, acetone solution, and absolute ethanol solution for 10 minutes, and finally wash it three times with distilled water.
[0062] 3. Preparation of 1.0M KOH solution
[0063] (1) Measure about 50mL of ultrapure water and 5.61g of potassium hydroxide, dissolve them with ultrapure water and stir, after cooling, dilute to volume in a 100mL volumetric flask.
[0064] 4. Preparation of amorphous (Ni,Fe)OOH films supported on the surface of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com