Polyhydroxy-substituted aromatic Schiff base synergistic halogen-free and flame-retardant nylon 6 composition and preparation method thereof
A flame-retardant nylon and polyhydroxyl technology, which is applied in the field of polymer material modification, can solve the problems of nylon 6 mechanical properties degradation, low flame-retardant efficiency, and tensile strength decline, and achieve the effect of blocking heat and oxygen transfer and blocking Good, good effect of cross-linked carbon layer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
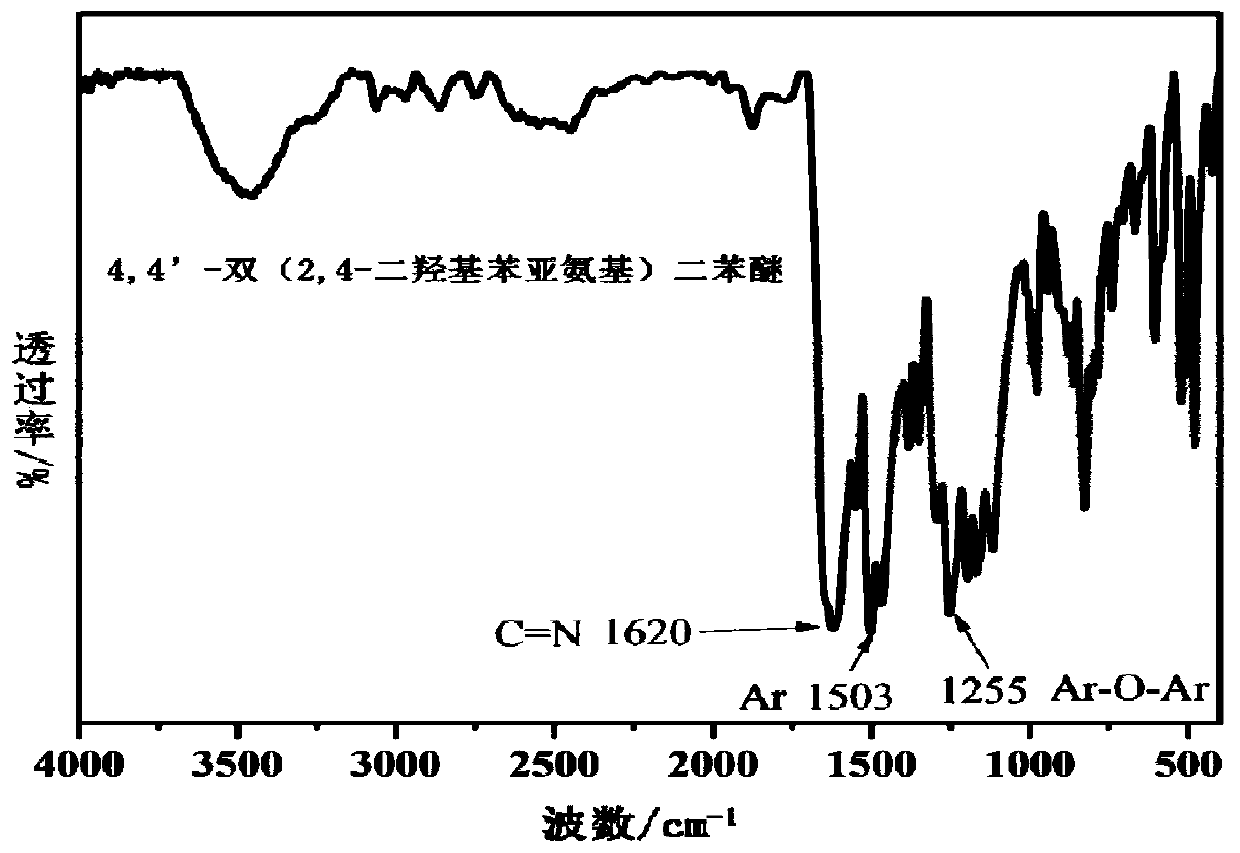
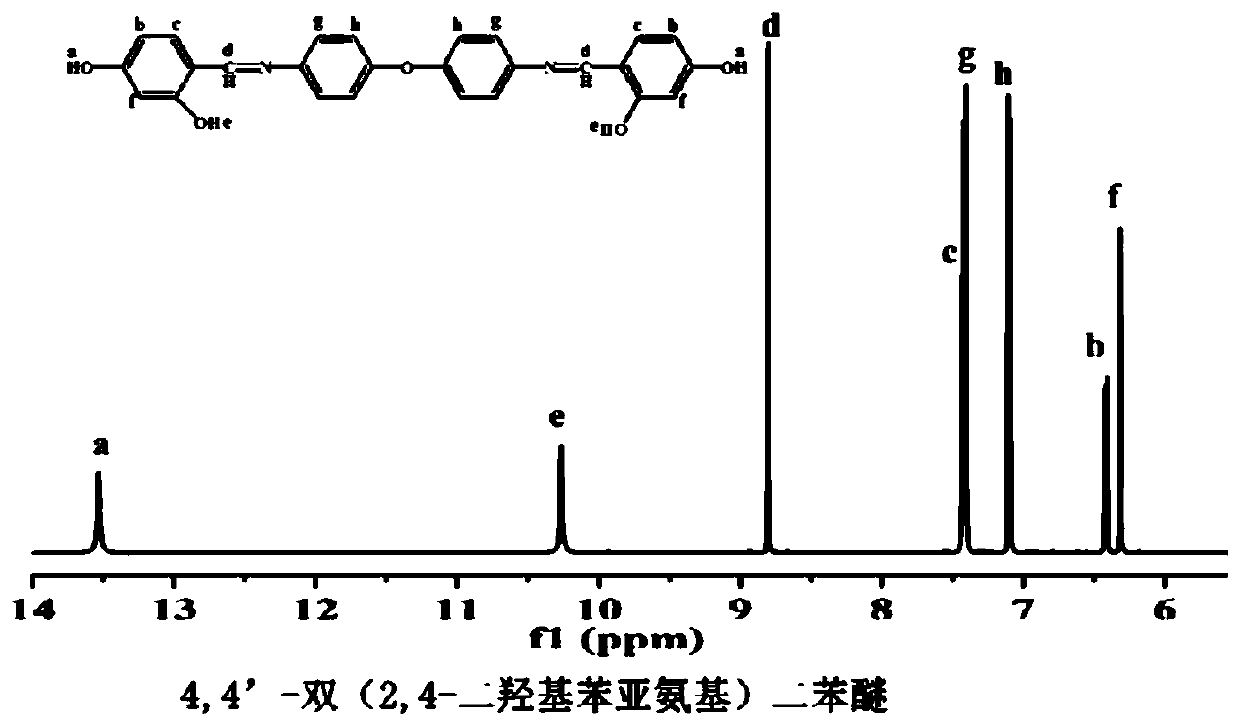
[0034] (1) Add 0.16mol 4,4'-diaminodiphenyl ether and 0.32mol 2,4-dihydroxybenzaldehyde into a 1000mL three-necked flask equipped with a magnet, a thermometer, and a nitrogen inlet tube, and then add 6.40mol of methanol solvent , magnetically stirred, and reacted at 60°C for 8h; the reaction liquid was cooled and filtered, washed with ethanol, and the filter cake was dried in a vacuum oven at 70°C for 11h to obtain an orange-yellow solid product, which was 4,4'-bis(2,4 -dihydroxyphenylimino) diphenyl ether, the yield is 92%; the infrared spectrum of the product is as attached figure 1 , 3100~3600cm on the spectrum -1 There is a broad characteristic absorption peak corresponding to the phenolic hydroxyl group, 1620cm -1 Corresponding to the characteristic peak of stretching vibration of imine bond (C=N), 1505cm -1 corresponding to the stretching vibration peak of the benzene ring (Ar), 1255cm -1 corresponding to the stretching vibration peak of the phenyl ether bond (Ar-O-Ar...
Embodiment 2
[0037] (1) Add 0.16mol 4,4'-diaminodiphenyl ether and 0.40mol 2,4-dihydroxybenzaldehyde to a 1000mL three-necked flask equipped with a magnet, a thermometer, and a nitrogen inlet tube, then add 6.80mol 1, 4-dioxane solvent, magnetic stirring, react at 85°C for 6h; the reaction liquid is cooled and filtered, washed with ethanol, and the filter cake is dried in a vacuum oven at 85°C for 6h to obtain an orange-yellow solid product, which is 4,4 '-bis(2,4-dihydroxyphenylimino)diphenyl ether, the yield is 96%; the infrared spectrum, hydrogen nuclear magnetic resonance spectrum and molecular structural formula of the product are consistent with those of the product in step (1) of Example 1;
[0038] (2) 685g of nylon 6, 80g of 4,4'-bis(2,4-dihydroxyphenylimino)diphenyl ether produced in step (1), 150g of high degree of polymerization ammonium polyphosphate, 80g of POE-g- MAH compatibilizer, 3g antioxidant B225 and 2g stearic acid join in the high-speed mixer, after stirring and mixi...
Embodiment 3
[0040] (1) Add 0.16mol 4,4'-diaminodiphenyl ether and 0.48mol 2,4-dihydroxybenzaldehyde to a 1000mL three-necked flask equipped with a magnet, a thermometer, and a nitrogen inlet tube, and then add 9.60mol toluene Solvent, magnetically stirred, reacted at 95°C for 4h; the reaction liquid was cooled and filtered, washed with methanol, and the filter cake was dried in a vacuum oven at 50°C for 12h to obtain an orange-yellow solid product, which was 4,4'-bis(2, 4-dihydroxyphenylimino) diphenyl ether, the yield is 94%; the infrared spectrum, hydrogen nuclear magnetic resonance spectrum and molecular structure of the product are consistent with those of the product in step (1) of Example 1;
[0041] (2) 716g of nylon 6, 80g of 4,4'-bis(2,4-dihydroxyphenylimino)diphenyl ether produced in step (1), 120g of high degree of polymerization ammonium polyphosphate, 80g of POE-g- MAH compatibilizer, 2g antioxidant 1010 and 2g stearic acid join in the high-speed mixer, after stirring and mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com