Preparation method of sodium chlorate
A technology of sodium chlorate and crude sodium chlorate, applied in the direction of electrolysis process, electrolysis components, etc., can solve the problems of unsatisfactory effect of anti-caking substances, insufficient purity, and low purity, so as to shorten the concentration time, improve the purity, The effect of improving performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
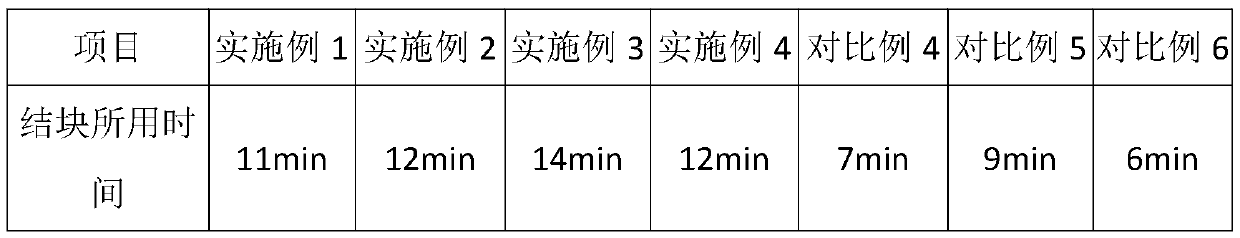
Examples
Embodiment 1
[0029] A kind of preparation method of sodium chlorate of embodiment 1
[0030] The preparation method of described sodium chlorate, comprises the following steps:
[0031] S1 Heat the water to 20°C, add salt to the water and stir until the salt in the water is saturated, then filter the salt water through a filter to obtain pure salt water;
[0032] S2 Add deionized water to the pure brine obtained in step S1 to dilute to obtain diluted brine, the volume ratio of the pure brine to deionized water is 5:1;
[0033] S3 adds the diluted brine obtained in step S2 into an electrolytic cell for electrolysis, with graphite as the anode and iron as the cathode, with a distance of 2 millimeters between the poles, and an electrolysis temperature of 45° C. to obtain a thick sodium chlorate solution;
[0034] S4 adds the ion chelating agent that is formed by sodium tripolyphosphate and sodium pyrophosphate by mass ratio 7:2 in step S3 gained thick sodium chlorate solution solution, leave...
Embodiment 2
[0038] A kind of preparation method of sodium chlorate of embodiment 2
[0039] A preparation method for sodium chlorate, comprising the following steps:
[0040] S1 Heat the water to 30°C, add salt to the water and stir until the salt in the water is saturated, then filter the salt water through a filter to obtain pure salt water;
[0041] S2 Add deionized water to the pure brine obtained in step S1 to dilute to obtain diluted brine, the volume ratio of the pure brine to deionized water is 5:3;
[0042] S3 adds the diluted brine obtained in step S2 into the electrolytic cell for electrolysis, with graphite as the anode, iron as the cathode, the distance between the poles is 3 millimeters, and the electrolysis temperature is 60° C. to obtain a thick sodium chlorate solution;
[0043] S4 adds the ion chelating agent that is formed by sodium tripolyphosphate and sodium pyrophosphate by mass ratio 9:4 in step S3 gained thick sodium chlorate solution solution, leaves standstill a...
Embodiment 3
[0047] A kind of preparation method of sodium chlorate of embodiment 3
[0048] A preparation method for sodium chlorate, comprising the following steps:
[0049] S1 Heat the water to 25°C, add salt to the water and stir until the salt in the water is saturated, then filter the salt water through a filter to obtain pure salt water;
[0050] S2 Add deionized water to the pure brine obtained in step S1 to dilute to obtain diluted brine, the volume ratio of the pure brine to deionized water is 5:2;
[0051] S3 adds the diluted brine obtained in step S2 into an electrolytic cell for electrolysis, with graphite as the anode and iron as the cathode, with a distance of 3 millimeters between the poles and an electrolysis temperature of 50° C. to obtain a thick sodium chlorate solution;
[0052] S4 adds the ion chelating agent that is formed by sodium tripolyphosphate and sodium pyrophosphate by mass ratio 8:3 in step S3 gained thick sodium chlorate solution solution, leaves standstil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com