Benzoxazole linked triphenylimidazole polymer as well as preparation method and application thereof
A technology of triphenylimidazole and benzoxazole, which is applied in the field of polymer and its preparation, and the polymer of benzoxazole linked with triphenylimidazole and its preparation, which can solve the problems of small specific surface area and weak adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
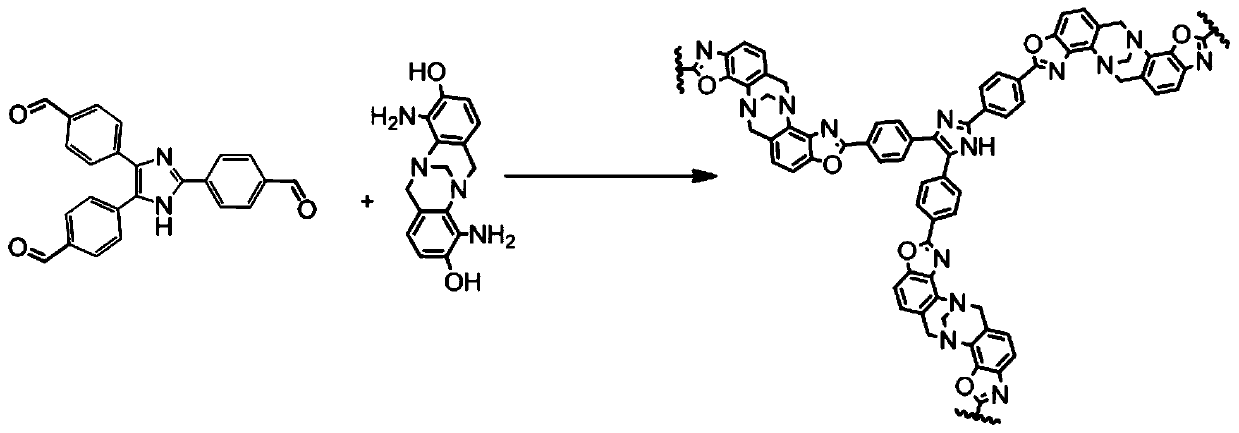
[0075] A kind of method for preparing the polymer of benzoxazole linking triphenylimidazole:
[0076] (1) Preparation of catalyst:
[0077] SiO 2 Place in a muffle furnace and activate at 200°C for 2h, then take 10g of activated SiO 2 Put in 100ml 10% NaHSO 4 Soak in water for 24 hours, suction filter, and dry to obtain the silica catalyst loaded with sodium bisulfate;
[0078] (2) Preparation of 4-(1,3-dithiolanyl)benzaldehyde (2):
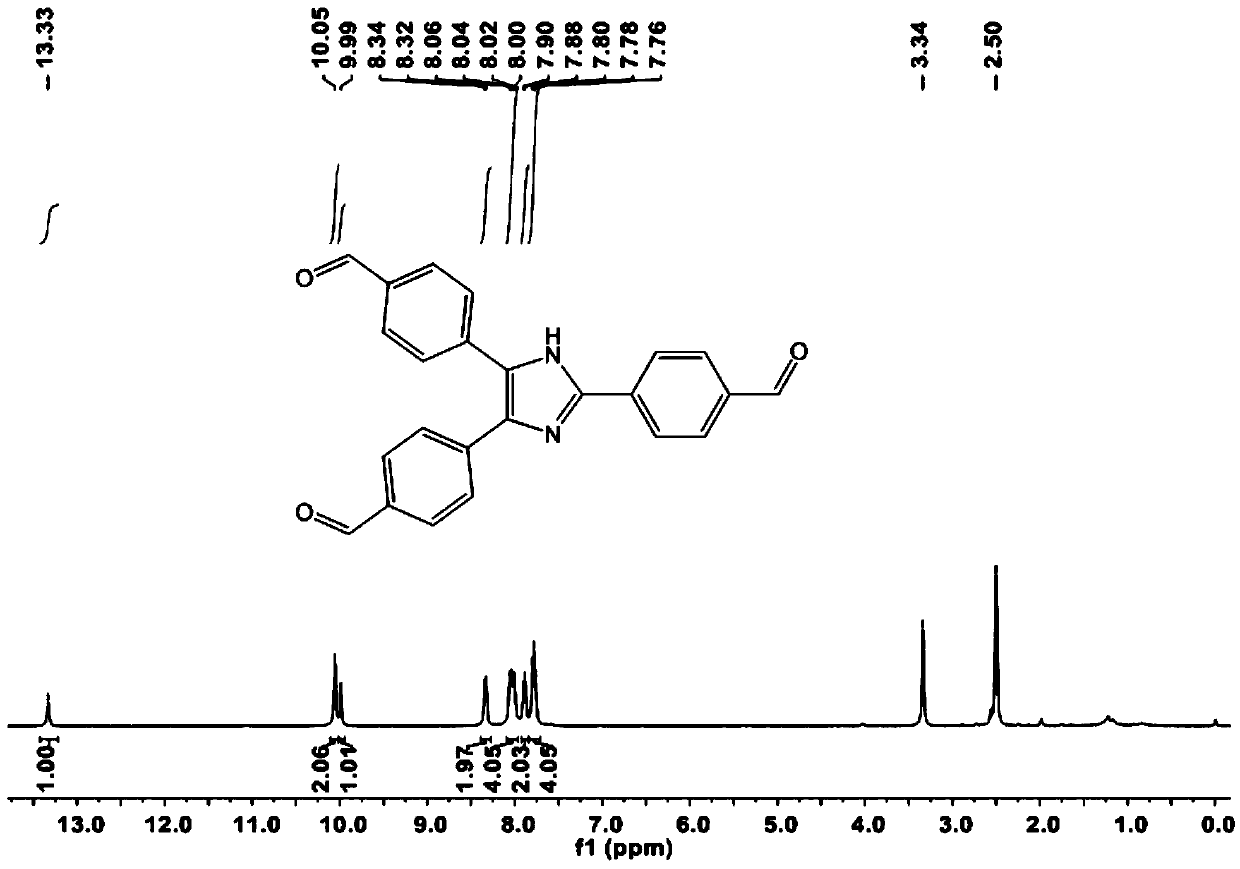
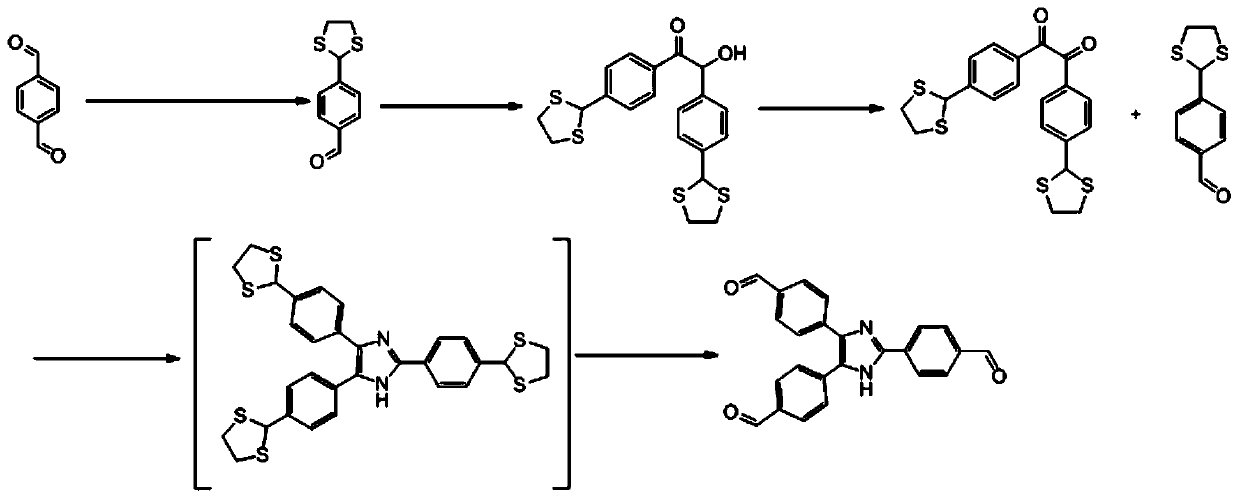
[0079] Dissolve 1g of terephthalaldehyde (1) in 10ml of dichloromethane, add 0.2g of the catalyst prepared in step (1), then slowly drop in 0.35g of ethanedithiol while stirring, at room temperature The reaction was stirred, and after the reaction was completed, the product 4-(1,3-dithiolanyl)benzaldehyde was separated by column chromatography (petroleum ether / ethyl acetate (10:1));
[0080] (3) Preparation of 1,2-bis(4-(1,3-dithiolanyl)phenyl)-2-hydroxyethanone (3):
[0081] Dissolve 2g of 4-(1,3-dithiolanyl)benzaldehyde in 10ml of 95% eth...
Embodiment 2
[0091] Repeat Example 1, but after the obtained benzoxazole-linked triphenylimidazole polymer is washed successively with acetone, water, 1M HCl, 1M NaOH, then in acetone:dichloromethane solution with a volume ratio of 1:1 After soaking in medium for 24 hours and suction filtration, vacuum drying at 120° C. for 24 hours yielded 490 mg (87%) of the final polymer.
Embodiment 3
[0093] A kind of method for preparing the polymer of benzoxazole linking triphenylimidazole:
[0094] (1) Preparation of catalyst:
[0095] Take 100g SiO 2 Place in a muffle furnace and activate at 200°C for 2h, then place the activated SiO 2 Pour 10% NaHSO 4 Soak in the solution for 24h to ensure that NaHSO 4 Adsorbed on SiO 2 Finally, filter through a Buchner funnel, transfer to a 60°C drying oven, and dry to obtain a silica catalyst loaded with sodium bisulfate;
[0096] (2) Preparation of 4-(1,3-dithiolanyl)benzaldehyde (2):
[0097] Add 1g (7.5mmol) of terephthalaldehyde, 10ml of anhydrous dichloromethane, 0.2g of silica catalyst loaded with sodium bisulfate into a 25ml round bottom flask, then slowly drop in 0.7g (7.5mmol) while stirring 1,2-ethanedithiol, the reaction was stirred at room temperature, followed by TLC until the reaction was completed. After the reaction was completed, 1.2 g of the product 4-(1,3-dithiolanyl)benzaldehyde was obtained by column chrom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com