Beta-ketone enamine chiral COFs (covalent organic frameworks) as well as preparation method and application of bonded capillary gas chromatographic column prepared from same
A technology of covalent organic framework and capillary column, which is applied in the direction of separation methods, chemical instruments and methods, and other chemical processes, and achieves the effects of being conducive to popularization and application, high thermal stability, and saving synthetic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
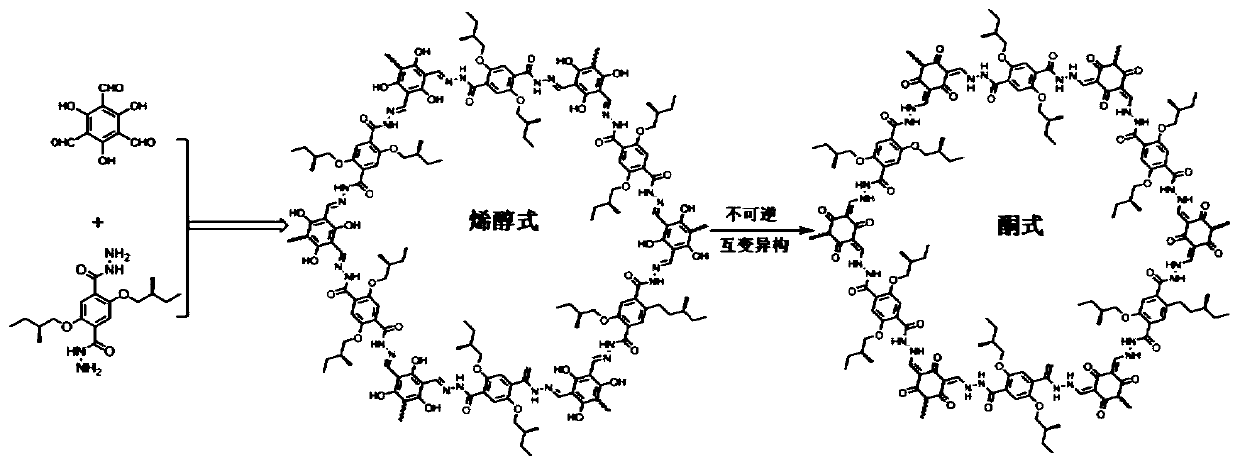
[0049] Example 1: Synthesis of β-ketoenamine chiral covalent organic framework materials
[0050] The chiral precursors (S)-2,5-bis(2-methylbutoxy)terephthalic acid dihydrazide (22.0 mg, 0.06 mmol), 2,4,6-trihydroxy-triphenylenetricarbaldehyde ( 8.4mg, 0.04mmol), 0.5mL of dioxane and 0.5mL of mesitylene were placed in a pressure-resistant reaction flask, mixed well and then added with 0.1mL of 3M acetic acid solution. The pressure-resistant reaction bottle was replaced with argon three times, and then quickly sealed with a sealing cap, and then placed in an oven preheated to 110° C. for 3 days. After the reaction, cool to room temperature, collect the solid by suction filtration, wash with 1,4-dioxane, tetrahydrofuran and acetone in sequence, and finally place and vacuum dry to obtain 25 mg of orange-yellow solid powder with a yield of 84%.
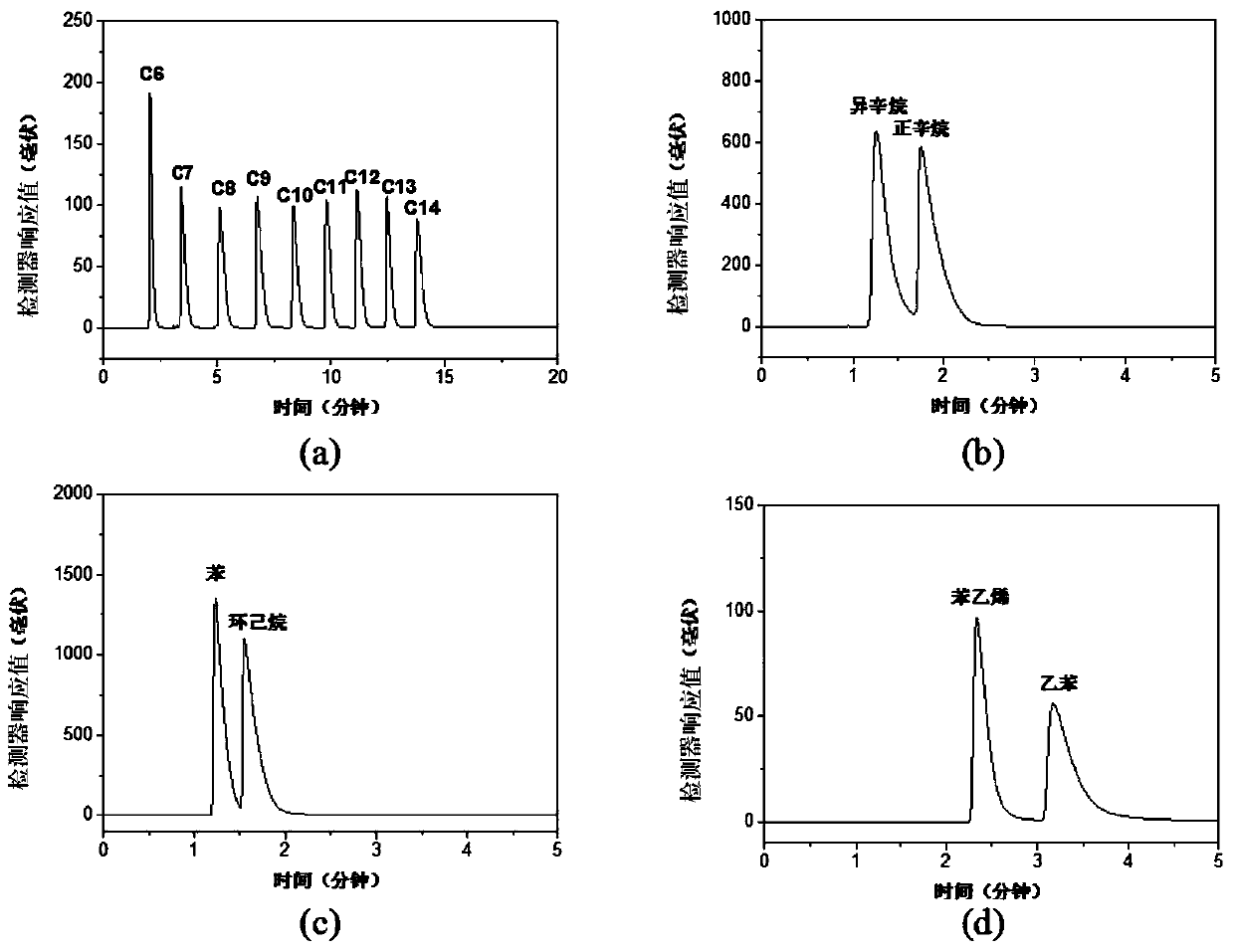
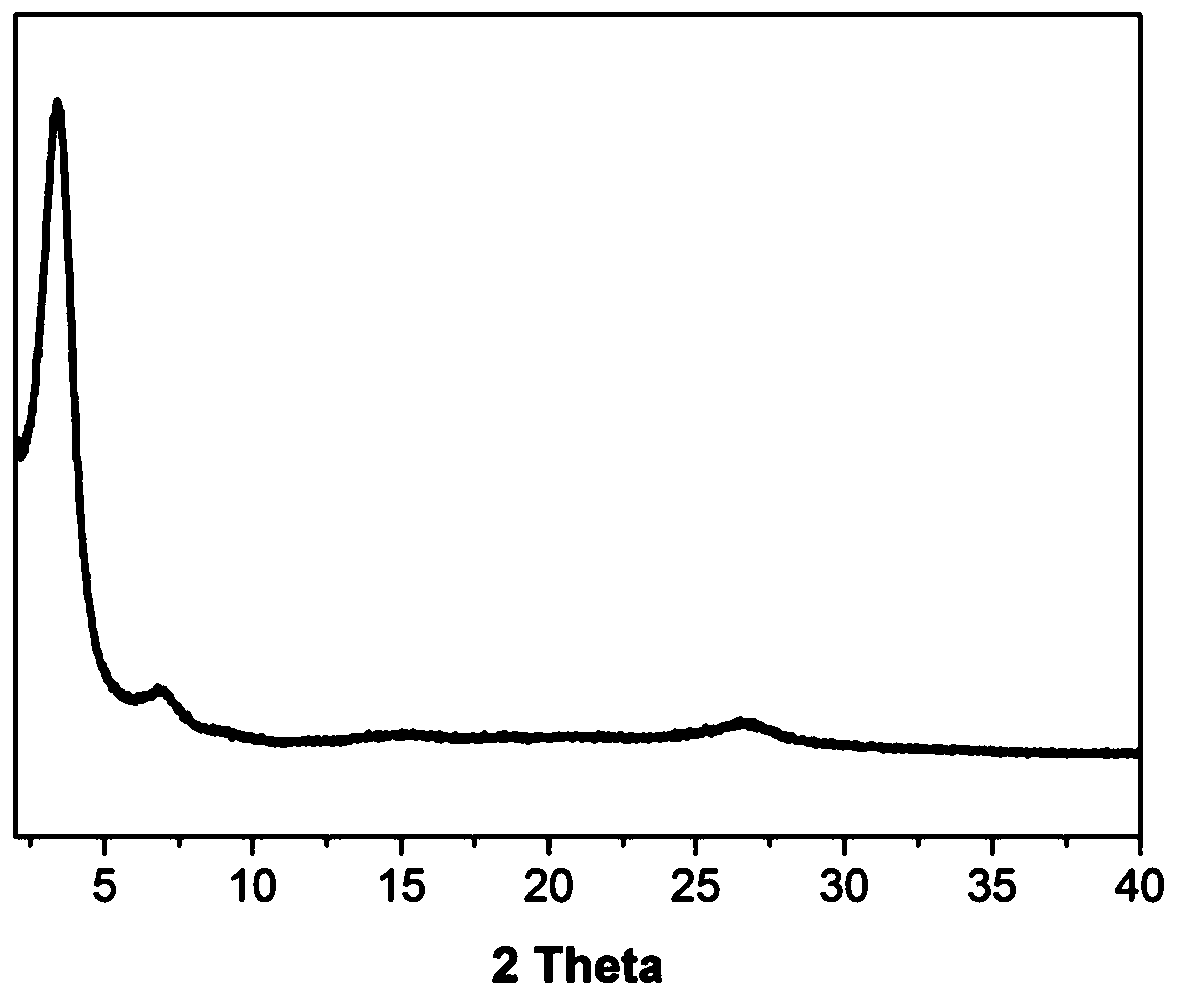
[0051] The performance measurement results of the hydroxyl functional group-containing β-ketoenamine chiral covalent organic framework ma...
Embodiment 2
[0060] Example 2: Preparation of bonded β-ketoenamine chiral covalent organic framework capillary column
[0061] (1) Pretreatment of capillary column: Quartz capillary column (10m×0.32mm) was washed with 1M sodium hydroxide solution for 2 hours, rinsed with deionized water for 30 minutes, then rinsed with 0.1M hydrochloric acid for 2 hours, and then used Rinse with deionized water until the eluate is neutral, then rinse with methanol for 30 minutes, and then fill the above-treated capillary with a methanol solution of 3-aminopropyltriethoxysilane (APTES) (50%, v / v) , both ends of the capillary were sealed with rubber stoppers and placed in a 40°C water bath overnight, then the residual solvent was flushed out with methanol, and finally, under the protection of nitrogen, the temperature was raised to 120°C and kept for 6 hours to dry the capillary column, thus obtaining the amino-modified Capillary, spare.
[0062] (2) Under ice-water bath conditions, 2,4,6-trihydroxy-pyrylen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com