A mutant of alginate lyase and its application
A technology of alginate lyase and mutants, which is applied in the field of alginate lyase mutants and its application, can solve the problems of poor substrate specificity and low enzyme activity, achieve strong pH stability and retain biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Construction of alginate lyase mutant E226K
[0026] Alginate lyase gene in the present invention AlgL (The nucleotide sequence is shown in SEQ ID NO.1) derived from the seawater isolated from Zhoushan Islands Pseudoalteromonas sp. zb7-4, with a full length of 1203 bp, encodes 400 amino acids, the 1st-31st amino acid is a signal peptide, the 32-131st amino acid is a carbohydrate binding domain (i.e. the CBM domain), and the 197th-385th amino acid is a catalytic domain. Truncating between amino acids 157-158, truncating amino acids 1-157 to obtain alginate lyase truncation enzyme AlgL-T157N, the sequence of which is shown in SEQ ID NO.3.
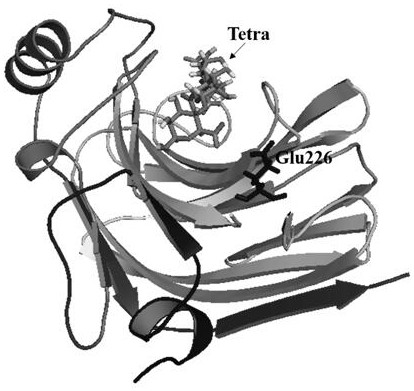
[0027] Through sequence comparison, it was found that AlgL-T157N and 4Q8K have the same amino acid sequence in the catalytic domain, so the docking results were combined based on the research on the protein structure and catalytic mechanism of 4Q8K ( figure 1 ), using Discovery Studio2016 software to perform virtual ala...
Embodiment 2
[0035] Example 2 Induced expression and purification of truncation enzyme AlgL-T157N and mutant E226K
[0036] Extract the recombinant plasmid pET-22b(+)- AlgL-T157N and pET-22b(+)- E226K , transfer them into E. coli From BL21(DE3) competent cells, pick positive recombinants in 5 mL LB (Amp + ) liquid medium, cultured at 200 r / min at 37°C for 12 h, and then inoculated to 25 mL LB (Amp + ) culture medium, 37℃ 200 r / min culture OD 600 Between 0.6-0.8, IPTG (final concentration 0.2 mmol / L) was added to induce at 20°C 200 r / min for 24 h, and the activity of alginate lyase was determined. The LB medium without IPTG after the same inoculation was used as a blank control. The enzyme activity of mutant E226K was 7.14±0.09 U / mL, which was 1.11 times higher than that of AlgL-T157N (3.38±0.11 U / mL).
[0037] The activity of alginate lyase was determined by DNS method. Add 0.1 mL of enzyme solution to 0.9 mL of 0.3% sodium alginate substrate, incubate at 50°C for 15 min, add 1...
Embodiment 3
[0049] Example 3 Separation and purification of truncation enzyme AlgL-T157N and mutant E226K
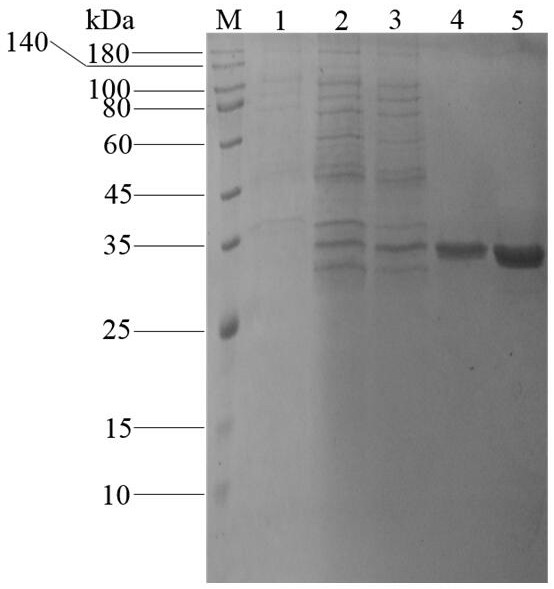
[0050] (1) DEAE anion column purification: use AKTA purification system to purify protein, first use DEAE FF (HiTrap TM , 5 mL) prepacked column to purify the protein, the column was equilibrated with McIlvaine buffer with a concentration of 50 mmol / L and pH 7.0 at a flow rate of 5 mL / min, and the crude enzyme solution was filtered with a 0.22 μm filter membrane. Load the obtained filtrate onto the anion column, and then equilibrate the column at a flow rate of 2 mL / min. After equilibrating for 4-5 column volumes, use the pre-prepared 1 mol / L NaCl solution (50mmol / L, McIlvaine buffer solution with pH 7.0 Preparation) Gradient elution column, detect protein by UV detector, collect protein peaks under different gradient elution, detect enzyme activity, and perform SDS-PAGE to detect protein purity.
[0051] (2) Nickel column purification: upload the protein preliminarily purified by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com