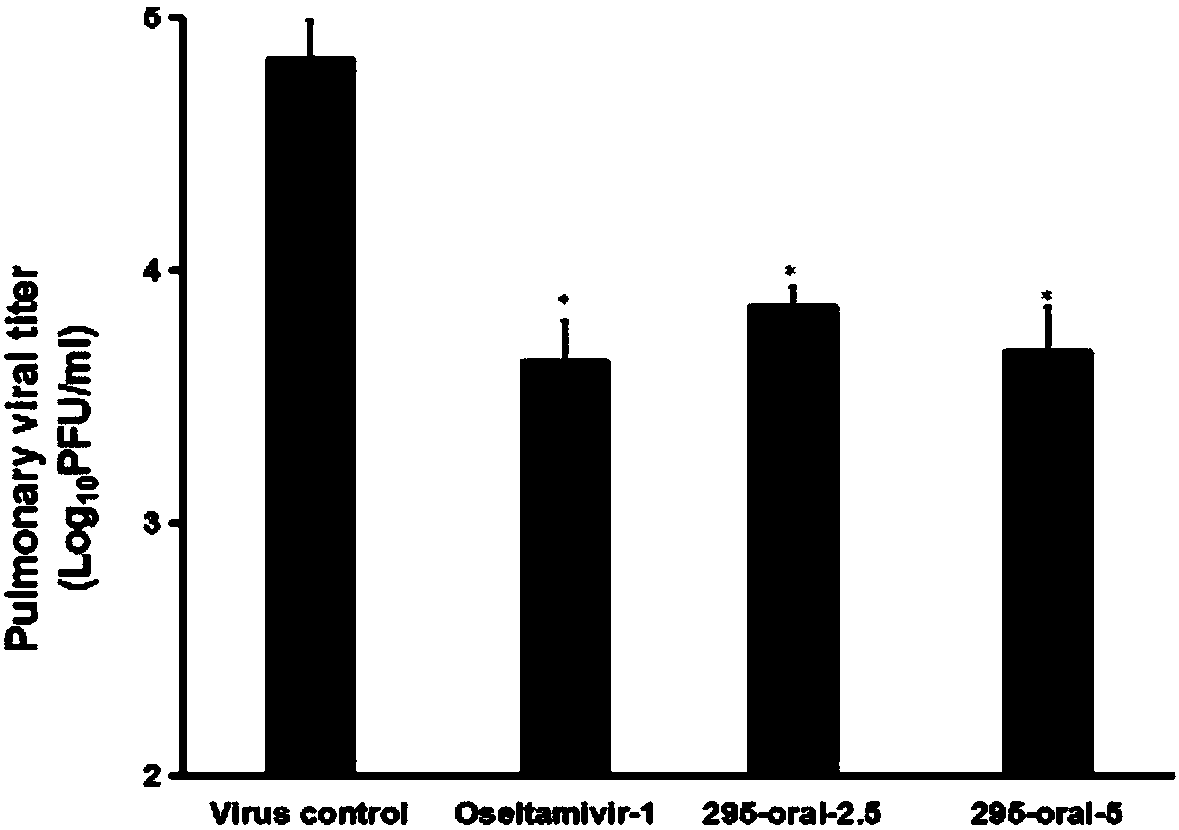
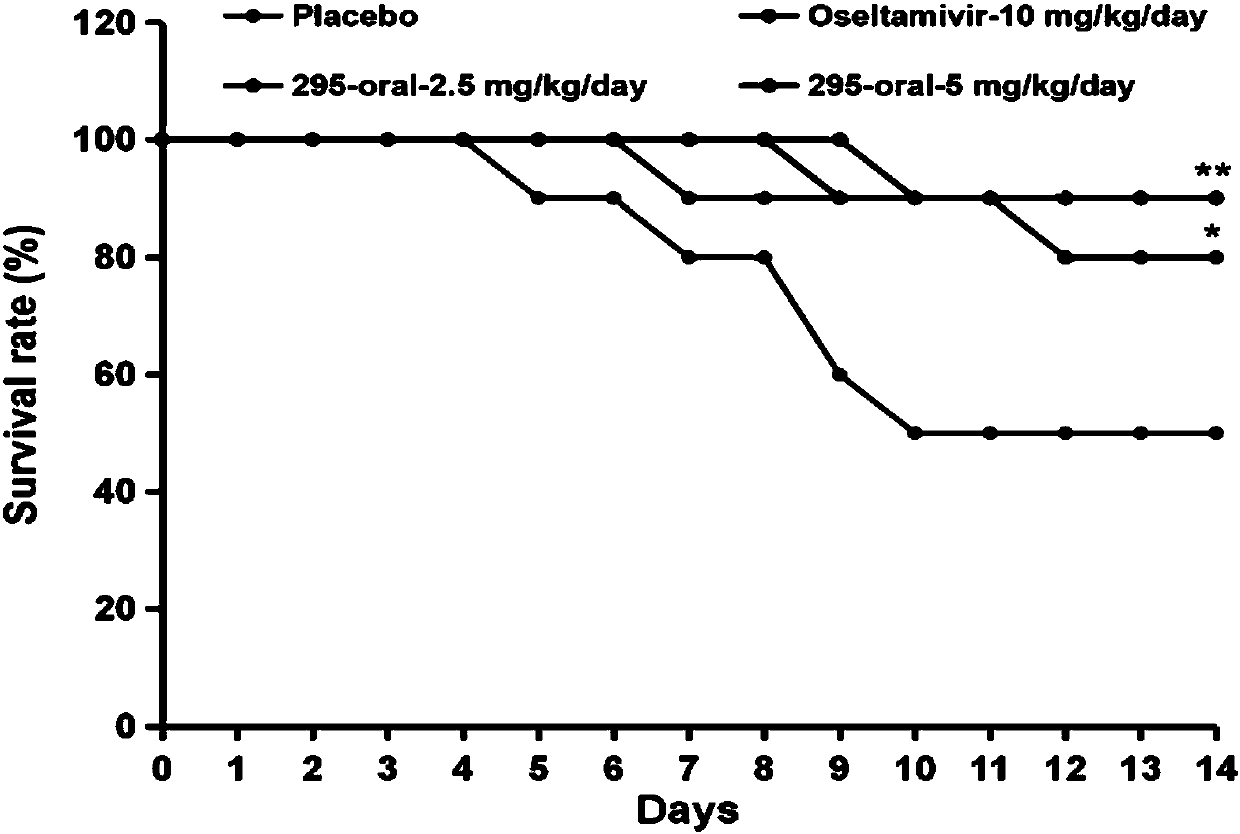
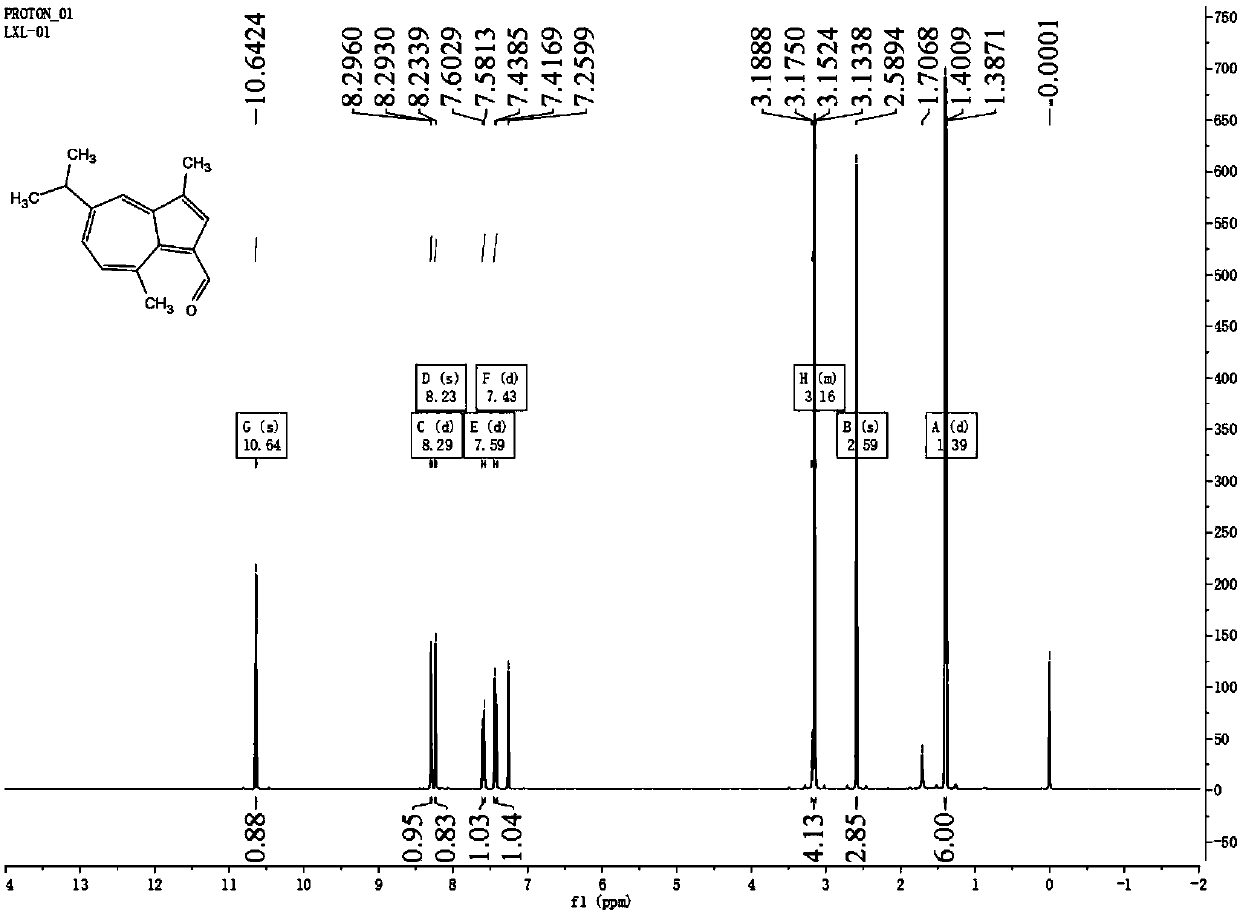
Preparation and application of trans-1,2-(1,4-diazulyl)ethene derivative
A technology of guaiac and azulene, which is applied in the field of chemical preparation and application of guaiac aldehyde dicondensate, can solve problems such as no reports of biological activity, and achieve the effect of enriching the library of antiviral active compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: the synthesis of azulene, reference (Takekuma S, Matsuoka H, Minematsu T et al. Tetrahedron, 2010, 66 (16): 3004-3015.) carry out the following operations:
[0018] Add dry 3mL DMF to a dry and clean 50mL three-necked round-bottom flask, slowly add phosphorus oxychloride dropwise through a constant pressure dropping funnel under ice bath conditions, and stir for 35min. Then add the DMF solution of guaiazulene dropwise through the dropping funnel from the other side, and transfer to a 35°C water bath after adding. After reacting for 10 h, the reaction solution was poured into a sufficient amount of ice water and stirred evenly. The pH of the reaction solution was adjusted to about 8 with an aqueous solution of potassium hydroxide. Stir for a few minutes, heat to 90°C, and drive out the dimethylamine gas generated by the reaction. Then it was extracted three times with dichloromethane, the combined extracts were evaporated to dryness, separated and purif...
Embodiment 2
[0019] Embodiment 2: the synthesis of pipecolic acid ethyl ester, reference (McFarlane A K, Thomas G, WhitingA.Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry, 1995, (21): 2803-2808. ) to perform the following operations:
[0020] Add 1.46g (10.0mmol) lysine to a 100mL round bottom flask, and add 50mL methanol. Thionyl chloride (1.5mL, 20.6mmol) was added dropwise while stirring at 0°C. After reacting for 3 hours, a sample was taken for TLC detection, and it was found that the lysine raw material was not completely reacted, and the reaction system was still a suspension. The reaction solution was transferred to an oil bath at 70°C, and after reflux for 4 hours, a sample was taken for TLC detection, and the lysine raw material disappeared. After the reaction was completed, the reaction solution was poured into 50 mL of ice water, adjusted to pH 6-7 with sodium hydroxide solution (10 mol / L), and evaporated to dryness to obtain a white s...
Embodiment 3
[0021] Embodiment 3: the synthesis of P2C
[0022] ① Fresh tert-butyl hypochlorite: add 5mL sodium hypochlorite solution (active chlorine content ≥ 5.2%, available chlorine ≥ 8.0mmol), 1mL (52.4mmol) tert-butanol in a 25mL round bottom flask, and place at 0°C Add 1 mL (17.2 mmol) of glacial acetic acid dropwise to the flask in a low-temperature constant-temperature stirring reaction bath, and react in the dark for about 5 minutes. After the reaction was completed, the reaction bottle was left to stand for 2 minutes, and the yellow oily liquid (ie tert-butyl hypochlorite) in the upper layer of the reaction bottle was absorbed, and placed in another light-proof glass vial for subsequent use.
[0023] ②N-Chlorination reaction: Add 500mg (2.6mmol) pipecolic acid ethyl ester hydrochloride into a 50mL round bottom flask, add 30mL anhydrous ether, ultrasonically dissolve (most of the materials are insoluble), then pump out the air in the bottle, quickly Seal the mouth of the bottle ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com