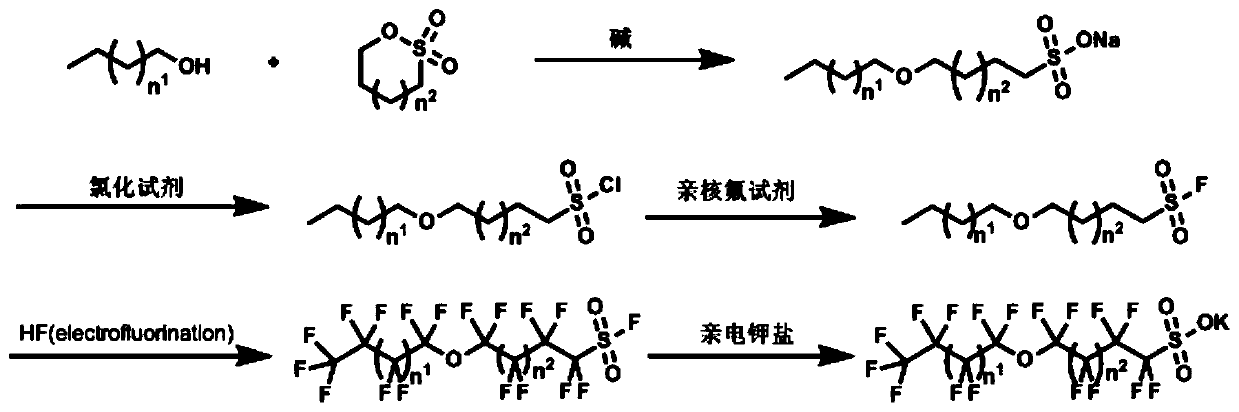
Perfluoroether type sulfuryl fluoride and sulfonate compounds and synthesis methods thereof
A technology of sulfonate-like compounds and sulfonyl fluoride compounds is applied in the field of perfluoroether sulfonyl fluoride and sulfonate compounds and their synthesis, which can solve the problem of high bioaccumulation and toxicity, low ether bond energy, and application range Limitation and other issues, to achieve the effect of low biological toxicity, low surface tension, and good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
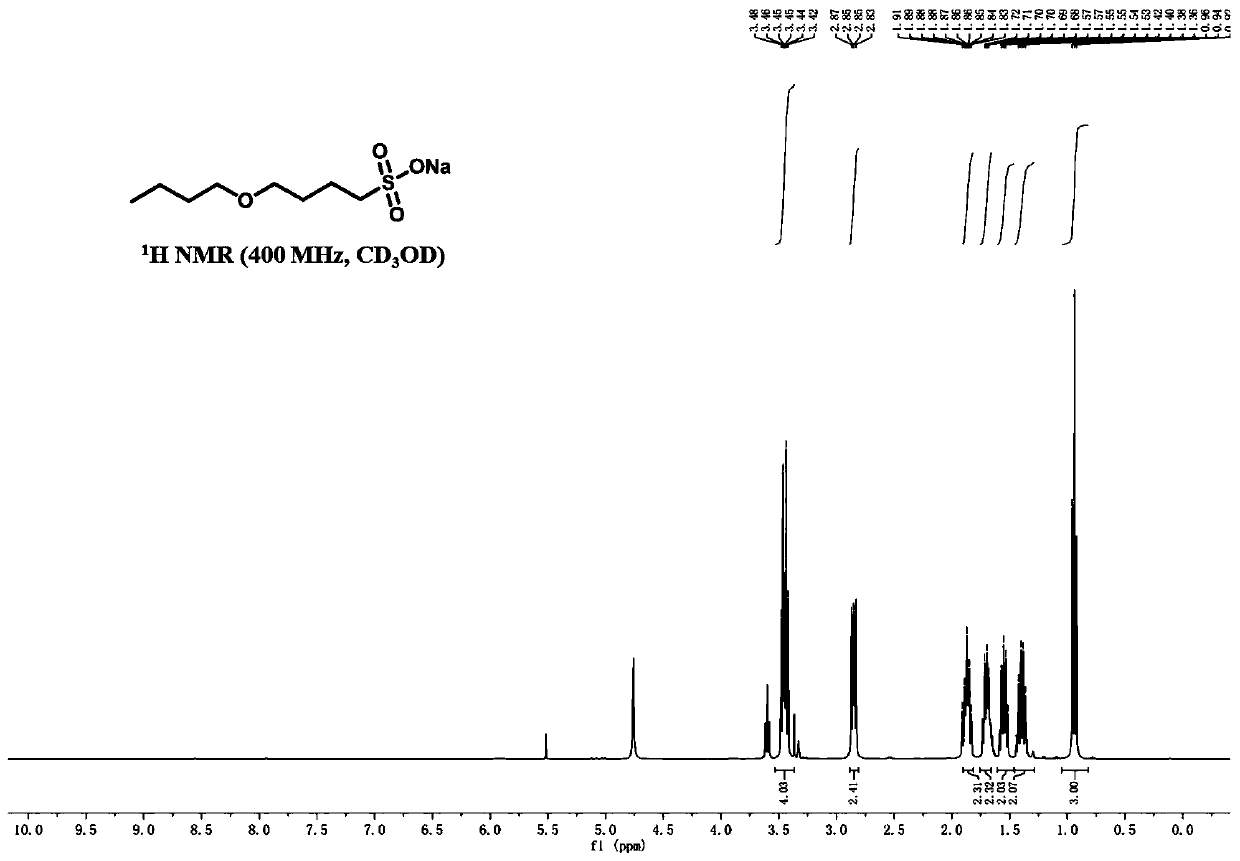
[0050] S1: Weigh 80g of n-butanol into a round bottom flask, add 250g (mass fraction: 22%) sodium hydroxide aqueous solution, heat up to 60°C, then add 150g of 1,4-butane sultone, and then heat up to 100 ℃, stirring the reaction for 6h. After the reaction, the volatile components were removed by distillation under reduced pressure, and the residue was dried to obtain the target product as a light yellow solid.
[0051] The target compound is detected by nuclear magnetic resonance, and the detected spectrum is as attached figure 2 As shown, the corresponding test result data is as follows: 1 H NMR (400MHz, CD 3 OD) δ3.46(t, J=12Hz, 2H), 3.44(t, J=12, 4.7Hz, 2H), 2.85(d, J=8.0Hz, 2H), 1.86(m, J=12Hz, 2H ), 1.71(m, J=6.6Hz, 2H), 1.68(m, J=8.4Hz, 2H), 1.40(m, J=4.8Hz, 2H), 0.95(t, J=4.8Hz, 3H). 13 C NMR (400MHz, CD 3 OD) δ71.2, 62.3, 52.2, 32.7, 29.6, 22.8, 20.2, 14.0.C 8 h 17 NaO 4 S, [M-Na] - The theoretically calculated value of 209.08475, high resolution mass spectro...
Embodiment 2
[0065] On the basis of Example 1, the 4-butoxyl-1-butylsulfonyl fluoride prepared in Example 1 is used to further prepare perfluoroether sulfonyl fluoride compounds. The specific steps are as follows:
[0066] S5: 5g of 4-perfluorobutoxy-1-perfluorobutylsulfonyl fluoride, 2g of potassium hydroxide and 2g of calcium oxide were added to 50mL of ethanol, and the temperature was raised to 50°C for 4 hours. After filtration, the obtained residue was precipitated under reduced pressure to obtain 4.8 g of the target compound as a white solid with a conversion rate of 90%.
[0067] The target compound is detected by nuclear magnetic resonance, and the detected spectrum is as attached Figure 7-8 As shown, the corresponding test result data is as follows: 13 C NMR (400MHz, CD 3 OD) δ119.1~118.5, 116.9~116.2, 115.8~114.0, 113.7~113.0, 111.8~111.1, 110.7~109.1, 108.8~107.4, 106.8~105.0.C 8 f 17 KO 4 S, [M-K] - The theoretical calculation value is 514.92458, and the high resolution ...
Embodiment 3
[0069] S1: Weigh 80g of n-butanol into a round bottom flask, add 250g (mass fraction: 22%) sodium hydroxide aqueous solution, heat up to 50°C, then add 150g of 1,4-butane sultone, and then heat up to 120°C ℃, stirring the reaction for 6h. After the reaction, the volatile components were removed by distillation under reduced pressure, and the residue was dried to obtain the target product as a light yellow solid.
[0070] The target compound was detected by nuclear magnetic resonance, and the corresponding detection result data are as follows: 1 H NMR (400MHz, CD 3 OD) δ3.48(t, J=12Hz, 2H), 3.45(t, J=12, 4.7Hz, 2H), 2.86(d, J=8.0Hz, 2H), 1.88(m, J=12Hz, 2H ), 1.73(m, J=6.6Hz, 2H), 1.69(m, J=8.4Hz, 2H), 1.42(m, J=4.8Hz, 2H), 0.96(t, J=4.8Hz, 3H). 13 C NMR (400MHz, CD 3 OD)δ71.3, 62.3, 52.3, 32.7, 29.7, 22.8, 20.2, 14.1.
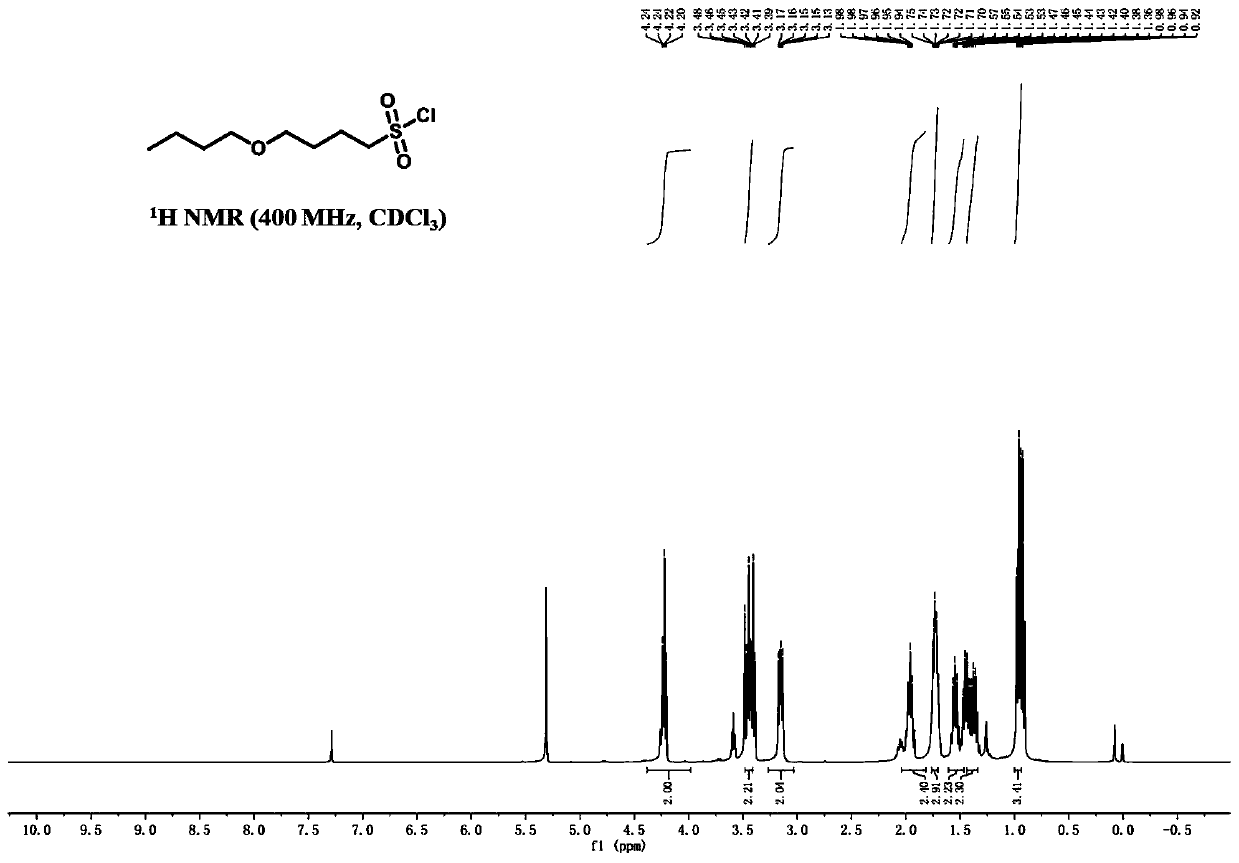
[0071] S2: Weigh 100g of sodium 4-butoxy-1-butylsulfonate into a round-bottomed flask, add 1L of chlorobenzene, raise the temperature to 40°C, add 322g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com