A composition for relieving joint pain and its medicine and application
A joint pain and composition technology, which is applied in the field of joint pain relief composition, can solve the problems of large side effects, etc., and achieve the effects of preventing degenerative changes, relieving pain, and promoting the improvement of joint activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: efficacy test
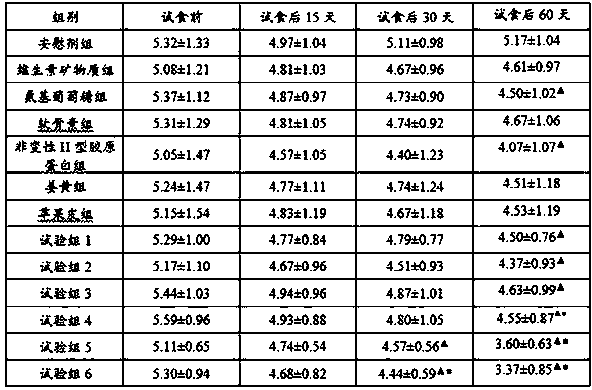
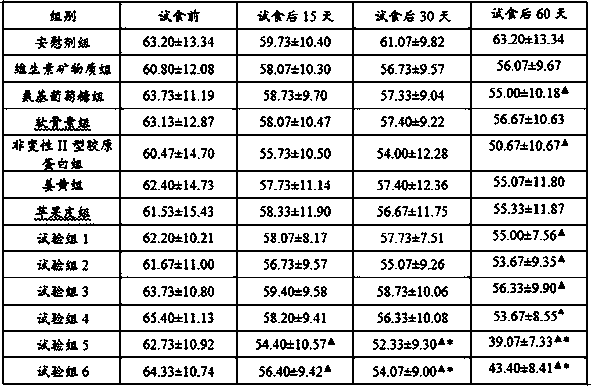
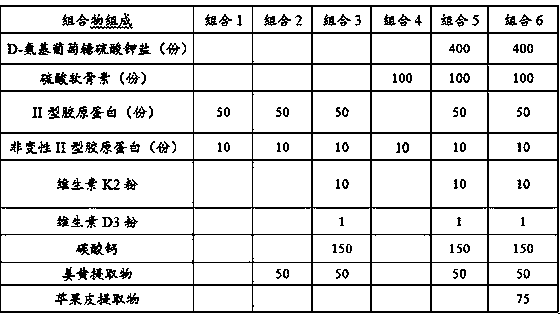
[0031] 1.1 The test groups are shown in Table 1 and Table 2:
[0032] Table 1: Combination 1~combination 5 formulations
[0033]
[0034] Table 2: Experimental group settings and intervention programs
[0035]
[0036] 2.2 Effect test:
[0037] 2.2.1 Test method
[0038]A total of 195 outpatients with knee arthritis and osteoarthritis were selected and randomized into 13 groups for double-blind intervention. The average W0MAC arthritis index score and VAS pain scale were used to evaluate the improvement of clinical symptoms and the changes in the structure and function of articular cartilage in each group before and after the intervention. situation and perform statistical analysis.
[0039] VAS Pain Rating Scale: This method uses a 10cm straight line or ruler, with the words 0 and 10 indicated at both ends, and the patient is asked to mark the corresponding position of their own pain or functional limitation on the straight line ...
Embodiment 2
[0049] Example 2: Preparation of film-coated tablets
[0050] 1. Product formula
[0051]
[0052] 2. The preparation process is as follows:
[0053] 2.1 Weighing and preparing materials:
[0054] Accurately weigh D-glucosamine sulfate potassium salt, calcium carbonate, chondroitin sulfate, turmeric extract, type II collagen, non-denatured type II collagen, vitamin K2 powder, vitamin D3 powder, and croscarmellose according to the formula quantity Sodium cellulose, microcrystalline cellulose, sodium carboxymethyl cellulose, silicon dioxide, magnesium stearate, and film coating premix for later use.
[0055] 2.2 Granulation and drying
[0056] ①Preparation of 0.5% sodium carboxymethyl cellulose slurry: add water in a certain proportion to prepare sodium carboxymethyl cellulose into 0.5% sodium carboxymethyl cellulose slurry for later use.
[0057] ② Add the formula amount of D-glucosamine sulfate potassium salt, calcium carbonate, and part of croscarmellose sodium into th...
Embodiment 3
[0065] Embodiment 3: the preparation of capsule
[0066] 1. Product formula
[0067]
[0068] 2. Preparation process:
[0069] 2.1 Weighing and preparing materials:
[0070] Accurately weigh D-glucosamine sulfate potassium salt, calcium carbonate, chondroitin sulfate, turmeric extract, type II collagen, non-denatured type II collagen, vitamin K2 powder, vitamin D3 powder, pregelatinized starch, Magnesium stearate and silicon dioxide are used for standby.
[0071] 2.2 Granulation and drying
[0072] ①Preparation of 0.5% sodium carboxymethyl cellulose slurry: add water in a certain proportion to prepare sodium carboxymethyl cellulose into 0.5% sodium carboxymethyl cellulose slurry for later use.
[0073] ② Add the formula amount of D-glucosamine sulfate potassium salt, calcium carbonate, and part of croscarmellose sodium into the granulator and mix, then start the boiling granulator, and transfer the materials to be granulated to the In the fluidized granulator, the prep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com