Cationic type covalent triazine framework material, preparation method and application thereof in iodine adsorption
A covalent triazine skeleton, cationic technology, used in chemical instruments and methods, adsorbed water/sewage treatment, other chemical processes, etc., can solve the problems of rare ionic CTFs material preparation and performance research.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
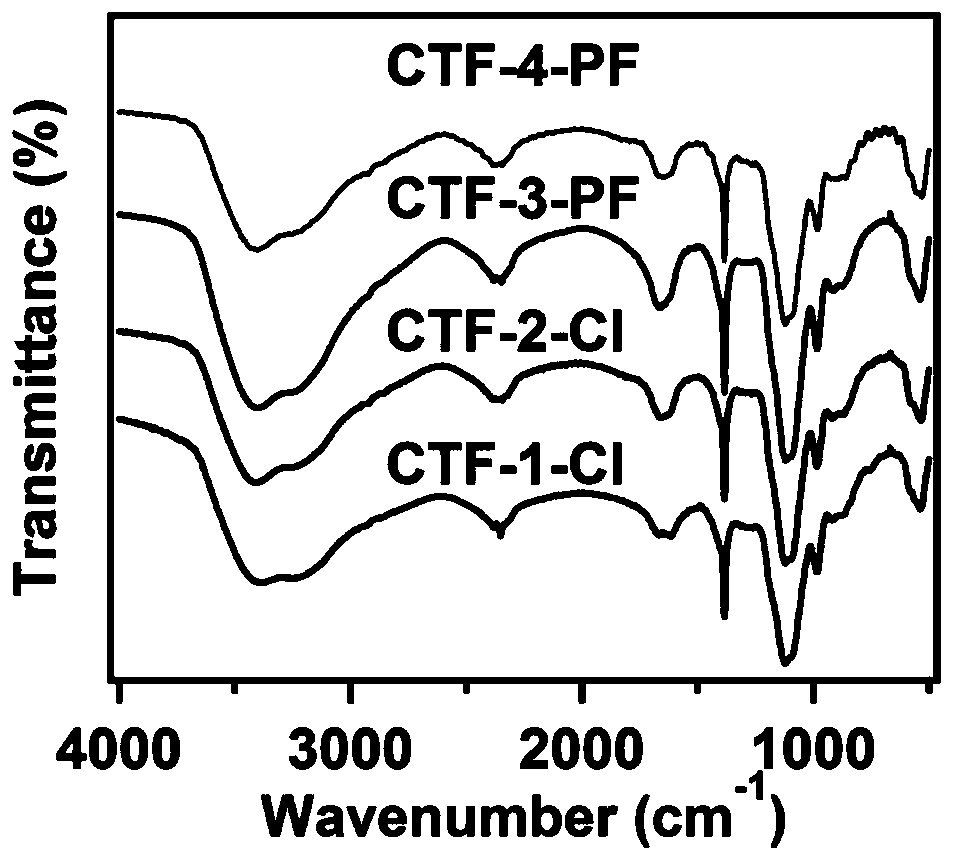
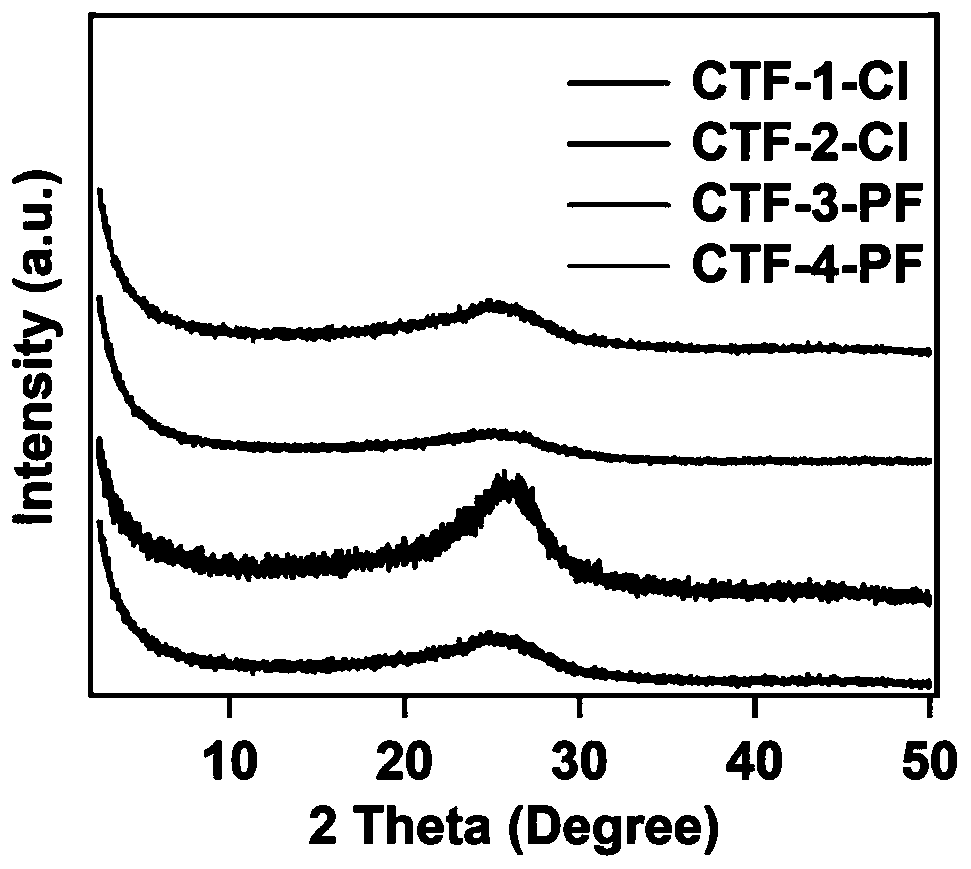
[0059] Embodiment 1 (preparation of CTF-1-Cl)
[0060] 1) Add 123mg of 1,3-bis(4-cyanophenyl)-1H-imidazolium-3-ammonium chloride and 273mg of zinc chloride into a quartz ampoule (3cm×12cm), and the molar ratio is 1: 5;
[0061] 2) Remove moisture and air in the ampoule under vacuum, and seal the ampoule;
[0062] 3) The ampoule is placed in a muffle furnace, the temperature is controlled at 400°C, and the reaction time is 48h;
[0063] 4) The ampoule is cooled down to room temperature, the tube is opened, and the reaction mixture is thoroughly washed with water to remove most of the zinc chloride, then further stirred in dilute HCl for 15 hours to remove residual salt, and then carried out with tetrahydrofuran and acetone respectively. Soxhlet extraction, removal of remaining impurities, and finally drying under vacuum gave CTF-1-Cl.
Embodiment 2
[0064] Embodiment 2 (preparation of CTF-2-Cl)
[0065] 1) Add 92mg of 1,3-bis(4-cyanophenyl)-1H-imidazolium-3-ammonium chloride and 409mg of zinc chloride into a quartz ampoule (3cm×12cm), and the molar ratio is 1: 10;
[0066] 2) Remove moisture and air in the ampoule under vacuum, and seal the ampoule;
[0067] 3) The ampoule is placed in a muffle furnace, the temperature is controlled at 400°C, and the reaction time is 48h;
[0068] 4) The ampoule is cooled down to room temperature, the tube is opened, and the reaction mixture is thoroughly washed with water to remove most of the zinc chloride, then further stirred in dilute HCl for 15 hours to remove residual salt, and then carried out with tetrahydrofuran and acetone respectively. Soxhlet extraction, removal of remaining impurities, and finally drying under vacuum gave CTF-2-Cl.
Embodiment 3
[0069] Embodiment 3 (preparation CTF-3-PF)
[0070] 1) Add 143mg of 1,3-bis(4-cyanophenyl)-1H-imidazolium-3-ammonium hexafluorophosphate and 273mg of zinc chloride into a quartz ampoule (3cm×12cm), and the molar ratio is 1 :5;
[0071] 2) Remove moisture and air in the ampoule under vacuum, and seal the ampoule;
[0072] 3) The ampoule is placed in a muffle furnace, the temperature is controlled at 400°C, and the reaction time is 48h;
[0073] 4) The ampoule is cooled down to room temperature, the tube is opened, and the reaction mixture is thoroughly washed with water to remove most of the zinc chloride, then further stirred in dilute HCl for 15 hours to remove residual salt, and then carried out with tetrahydrofuran and acetone respectively. Soxhlet extraction, removal of remaining impurities, and finally drying under vacuum yielded CTF-3-PF.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com