Preparation method of thioether compound
A technology of thioether compound and reaction, which is applied in the field of preparation of thioether compound, can solve the problems of being unfriendly to the human body or the environment, complicated reaction process, and many side reactions, and achieves favorable promotion and application, simple reaction process, and avoiding side effects. The effect of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
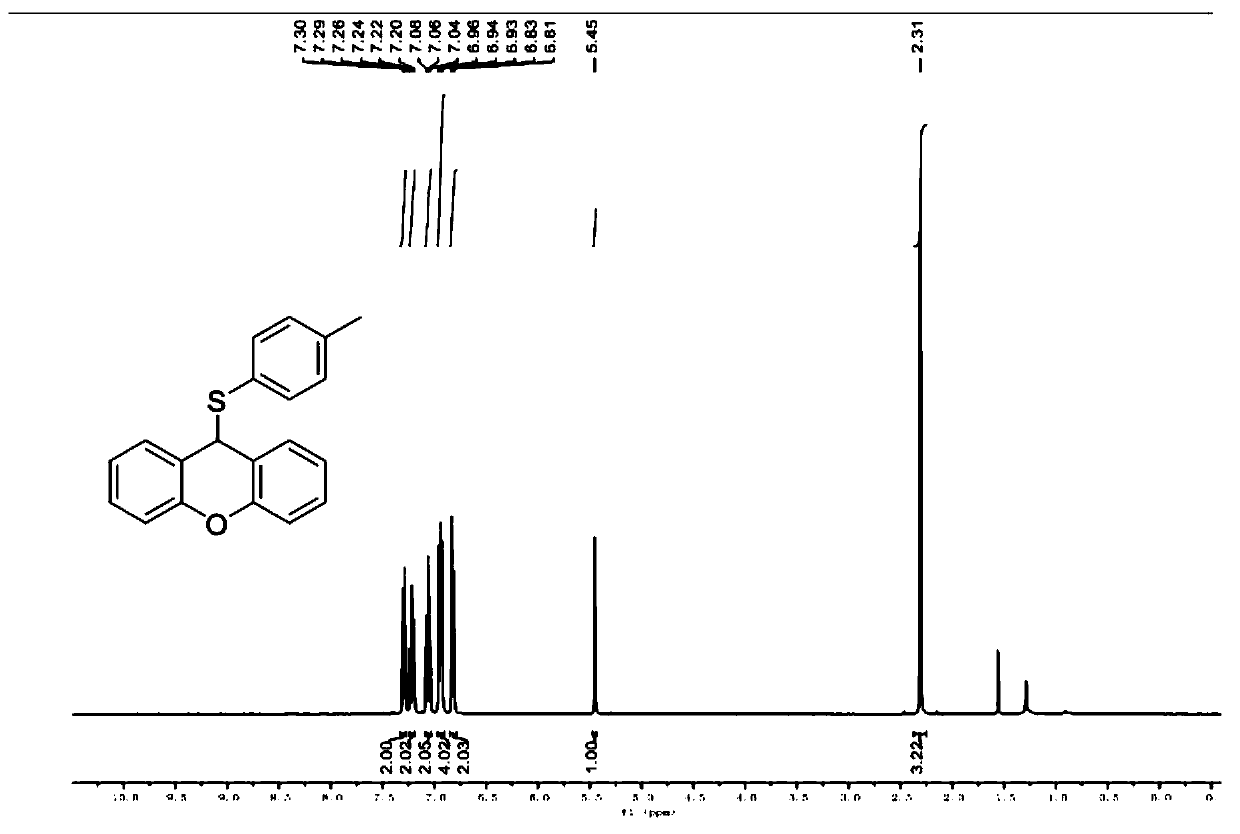
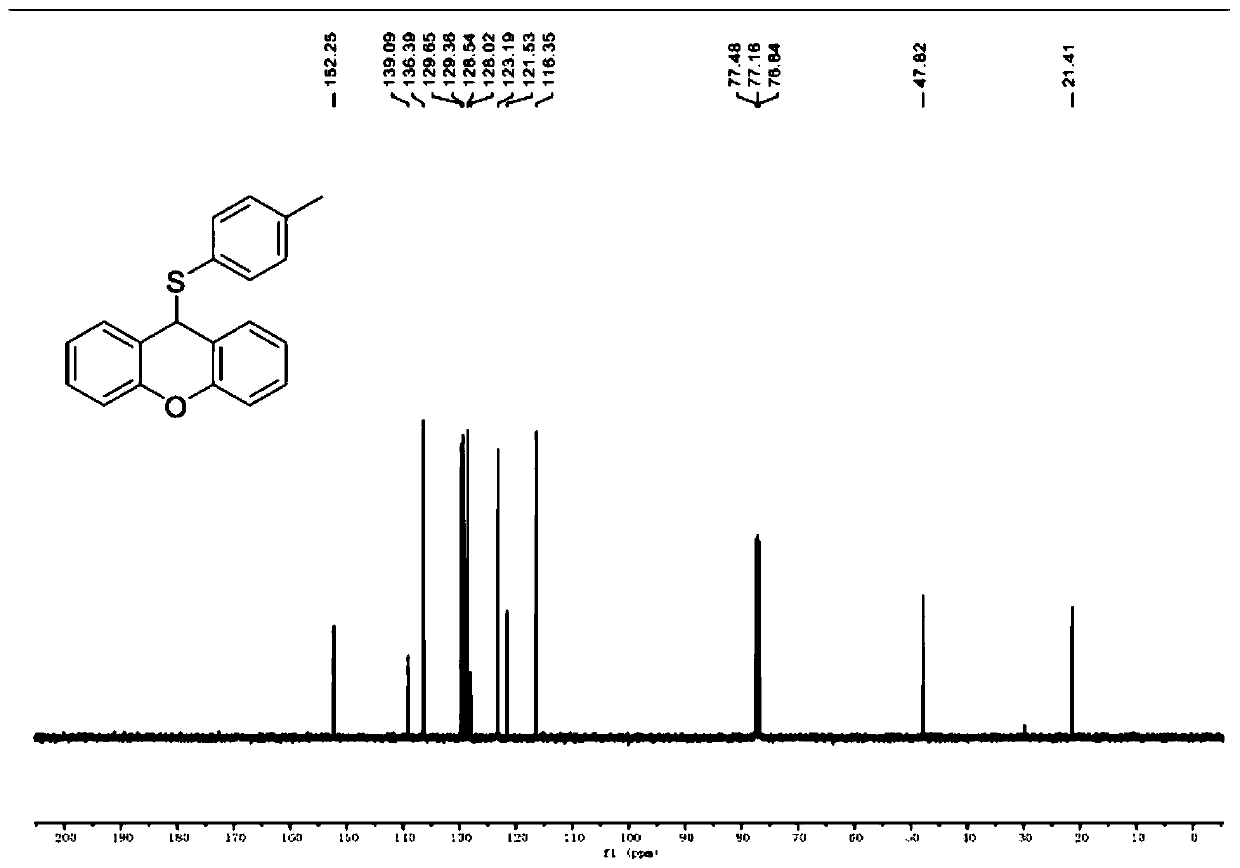
[0050] Example 1 Synthesis of 9-((4-methylphenyl)thio)-9H-oxanthene
[0051] The structure of 9-((4-methylphenyl)thio)-9H-xanthene is shown below:
[0052]
[0053]The specific preparation process is: put 49.6mg of p-methylthiophenol (0.4mmol) and 36.4mg of xanthene (0.2mmol) into 15mL Schlenk, add 2mL of absolute ethanol, magnetically stir at room temperature to completely dissolve, and monitor by TLC reaction process. After reacting at room temperature for 24 hours, the solvent ethanol was distilled off to obtain the crude product, and then a developing solvent with a volume ratio of petroleum ether / ethyl acetate of 100 / 1 was used to obtain 9-((4-methylphenyl) by column chromatography. Thio)-9H-xanthene, white solid. The yield of this step was 84% (51 mg).
[0054] Product NMR verification results are as follows (see figure 1 and figure 2 ).
[0055] 1 H NMR (400MHz, CDCl 3 )δ7.29(d,J=7.6Hz,2H),7.24–7.20(m,2H),7.08–7.04(m,2H),6.97–6.91(m,4H),6.82(d,J=8.0Hz ,2H...
Embodiment 2
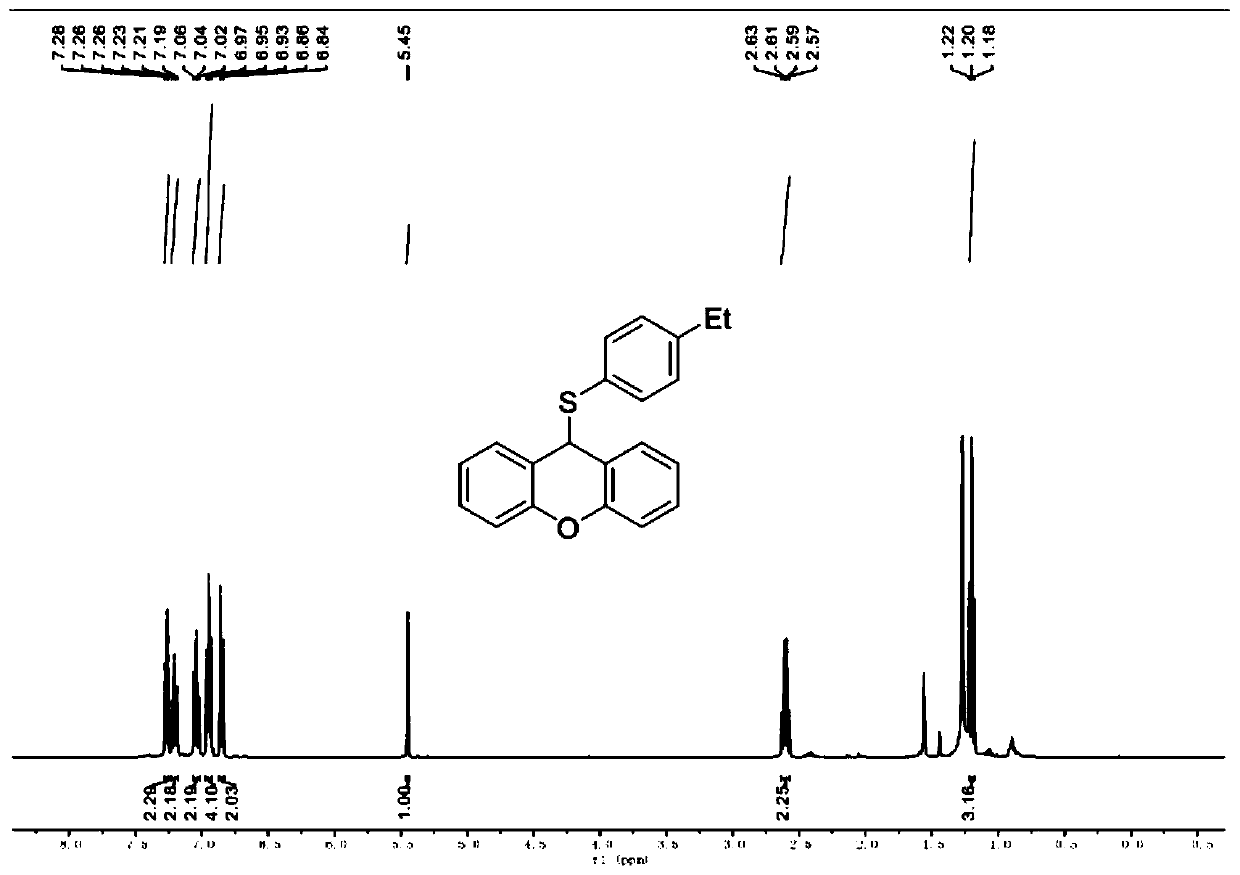
[0057] Example 2 Synthesis of 9-((4-ethylphenyl)thio)-9H-oxanthene
[0058] The structure of 9-((4-ethylphenyl)thio)-9H-xanthene is shown below:
[0059]
[0060] The specific preparation process is as follows: put 55.2mg of p-ethylthiophenol (0.4mmol) and 36.4mg of xanthene (0.2mmol) into 15mL Schlenk, add 2mL of absolute ethanol, stir magnetically at room temperature to completely dissolve, and monitor by TLC reaction process. After reacting at room temperature for 24 hours, the solvent ethanol was distilled off to obtain the crude product, and then a developing solvent with a volume ratio of petroleum ether / ethyl acetate of 100 / 1 was used to obtain 9-((4-ethylphenyl) by column chromatography. Sulfuryl)-9H-xanthene, colorless oily liquid. The yield of this step was 87% (55.3mg).
[0061] Product NMR verification results are as follows (see image 3 and Figure 4 ).
[0062] 1 H NMR (400MHz, CDCl 3 )δ7.29–7.25(m,2H),7.21(t,J=8.5Hz,2H),7.04(t,J=8.0Hz,2H),6.95(t,J=6....
Embodiment 3
[0064] Example 3 Synthesis of 9-((4-isopropylphenyl)thio)-9H-oxanthene
[0065] The structure of 9-((4-isopropylphenyl)thio)-9H-xanthene is shown below:
[0066]
[0067] The specific preparation process is as follows: put 60.8mg of p-isopropylthiophenol (0.4mmol) and 36.4mg of xanthene (0.2mmol) into 15mL Schlenk, add 2ml of absolute ethanol, stir magnetically at room temperature to completely dissolve, and pass TLC Monitor the progress of the reaction. After reacting at room temperature for 24 hours, the solvent ethanol was distilled off to obtain the crude product, and then the developing solvent with sherwood oil / ethyl acetate volume ratio of 100 / 1 was obtained by column chromatography to obtain 9-((4-isopropylphenyl )thio)-9H-xanthene, light yellow oily liquid. The yield of this step was 90% (59.7mg).
[0068] Product NMR verification results are as follows (see Figure 5 and Figure 6 ).
[0069] 1 H NMR (400MHz, CDCl 3 )δ7.26–7.19(m,4H),7.03(t,J=6.9Hz,2H),6.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com