Method for preparing protease 3 recombinant protein
A recombinant protein and protease technology, applied in the field of preparing protease 3 recombinant protein, can solve the problems of difficult separation and purification of natural protein, inconsistent between batches, low product purity, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Construction of recombinant donor vector pFastBac1-Mel-PR3-His containing insect secretion signal peptide Mel
[0040] PCR amplification primers were designed using the cDNA of human PR3 (GenBankNo: X55668.1) as a template, and Xho I and Kpn I restriction sites were introduced at the N-terminal and C-terminal of the human PR3 gene sequence, so as to ligate the fragments Into the plasmid vector after double enzyme digestion; its C-terminus introduces 6 bases encoding histidine to facilitate the purification of expressed protein: the primers are as follows:
[0041] Forward primer-1 (SEQ ID NO.1): AGGAGACTCGAGATCGTGGGCGGGCACGAGGCG; Reverse primer-1 (SEQ ID NO.2): TACGGTACCCTAGTGATGGTGATGGTGATGACGGCGCAGCGTGGAACG.
[0042] Using the above primers, the first round of PCR amplification reaction was carried out using the cDNA of human PR3 as a template. The reaction system and conditions are shown in Table 1:
[0043] Table 1. PCR amplification reaction system and ...
Embodiment 2
[0051] Example 2: Preparation of recombinant Bombyx mori baculovirus rBm-pFastBac1-Mel-PR3-His
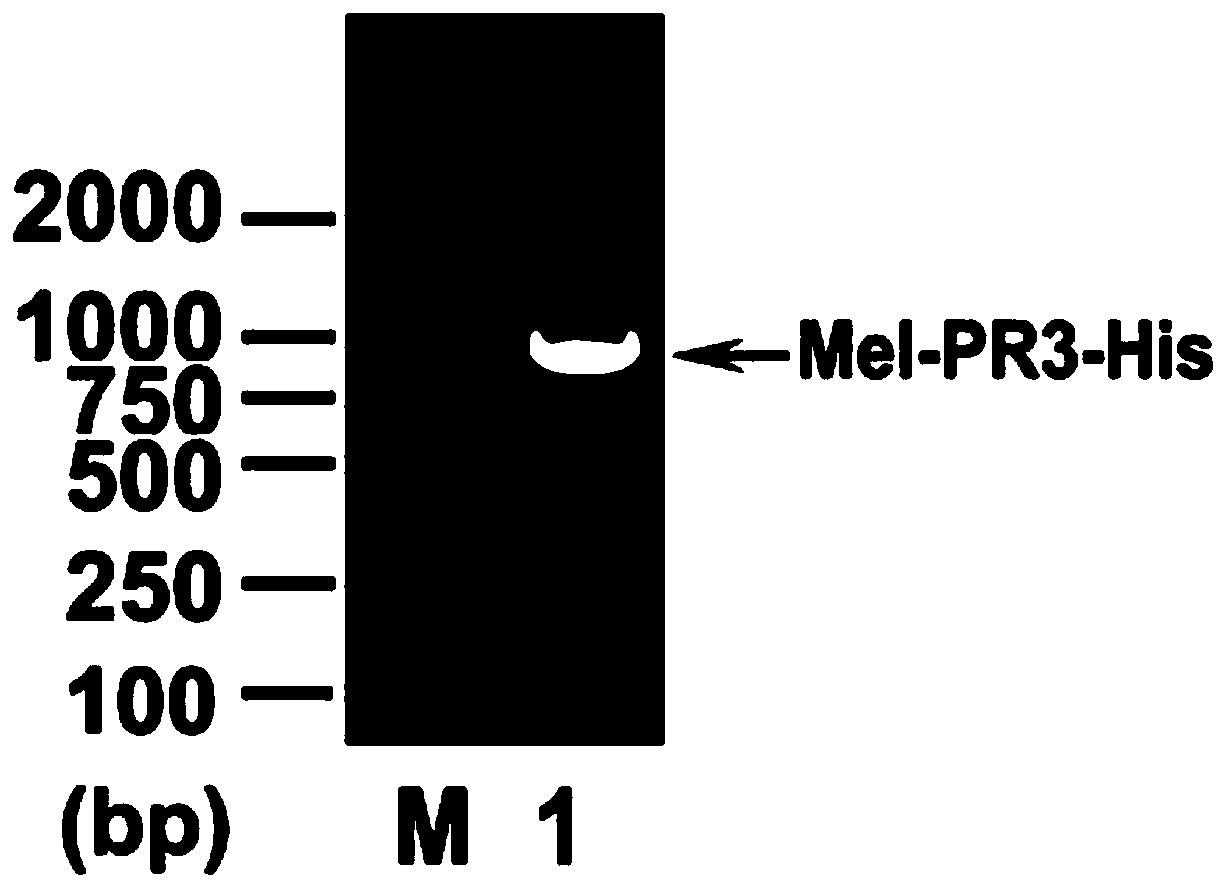
[0052] The recombinant donor vector pFastBac1-Mel-PR3-His constructed in Example 1 containing the insect secretion signal peptide Mel was transfected into Escherichia coli BmDH10Bac, the Tn7 transposable element contained in the vector pFastBac1-Mel-PR3-His and the silkworm BmNPV rod The recombined shuttle vector was identified after the transposition of Bacmid, a virus shuttle vector, was screened by blue and white. Randomly select 2 Bacmid DNA recombinant shuttle vectors of white spot, and use non-recombinant Bacmid DNA of blue spot as a control, apply specific universal pUC / M13 forward primer (SEQ ID NO.5): 5'-CCCAGTCACGACGTTGTAAAACG-3' and reverse Primer (SEQ ID NO.6): 5'-AGCGGATAACAATTTCACACAGG-3', used for PCR reaction verification of recombinant shuttle vector Bacmid DNA.
[0053] Figure 5 It is the result of PCR amplification of recombinant and non-recombinant shuttle ve...
Embodiment 3
[0055] Example 3: Expression of recombinant Bombyx mori baculovirus rBm-pFastBac1-Mel-PR3-His in fifth instar silkworm
[0056] The recombinant Bombyx mori baculovirus rBm-pFastBac1-Mel-PR3-His prepared in Example 2 was inoculated into silkworms on the second day after transfection to the fifth instar, and each silkworm was subcutaneously injected with 5 µL of recombinant Bombyx mori baculovirus rBm-pFastBac1 -Mel-PR3-His, that is: each silkworm was inoculated with 1.0×10 5PFU virus. After virus inoculation, silkworm hemolymph was collected every 24 hours. At the same time, non-virus-inoculated silkworms were used as a negative control group to collect hemolymph together with virus-inoculated silkworms. Human PR3 protein ELISA detection kit was used to collect 5 samples at different times. The mixed hemolymph of the head silkworm was used as a sample, and the ELISA activity of the human PR3 protein contained in the hemolymph and the human PR3 antibody coated on the microwell ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com