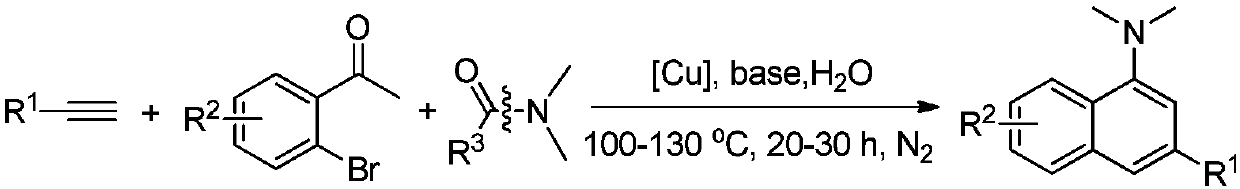
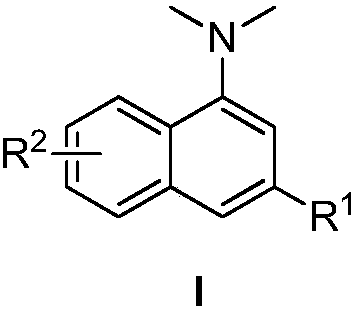
N,N-dimethyl-1-naphthylamine compound preparation method
A technology for dimethyl amide and compound, which is applied in the field of preparation of N,N-dimethyl-1-naphthylamine compounds, can solve the problems of harsh reaction conditions, low compound yield, inability to prepare and the like, and achieves reaction operation The effect of simplicity, high product yield and strong compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0026] Synthesis of N,N-Dimethyl-3-phenylnaphthalene-1-amine
[0027] Add 0.20mmol phenylacetylene, 0.40mmol o-bromoacetophenone, 0.30mmol N,N-dimethylformamide, 0.02mmol cuprous iodide, 0.40mmol sodium hydroxide, and 1.0mL water into the reactor. Under nitrogen atmosphere, heat to 120°C, keep stirring for 22h, stop the reaction, cool to room temperature, add saturated ammonium chloride solution to wash, then extract with ethyl acetate, dry, and distill off the solvent under reduced pressure, the crude product is separated by column chromatography The target product was obtained with a yield of 83%. 1 H NMR (400MHz, CDCl 3 ):δ8.26-8.23(m,1H),7.89-7.86(m,1H),7.74-7.72(m,3H),7.51-7.47(m,4H),7.39(t,J=7.4Hz,1H ), 7.32(s,1H), 2.97(s,6H).
Synthetic example 2
[0029] Synthesis of 3-(4-fluorophenyl)-N,N-dimethylnaphthalene-1-amine
[0030] Add 0.20mmol 4-fluorophenylacetylene, 0.40mmol o-bromoacetophenone, 0.30mmol N,N-dimethylformamide, 0.02mmol cuprous iodide, 0.40mmol sodium hydroxide, and 1.0mL water into the reactor. Under nitrogen atmosphere, heat to 120°C, keep stirring for 24h, stop the reaction, cool to room temperature, add saturated ammonium chloride solution to wash, then extract with ethyl acetate, dry, and distill off the solvent under reduced pressure, the crude product is separated by column chromatography The target product was obtained with a yield of 81%. 1 H NMR (400MHz, CDCl 3 ):δ8.25-8.23(m,1H),7.88-7.86(m,1H),7.69-7.66(m,3H),7.51-7.49(m,2H),7.25(s,1H),7.18(t , J=8.1Hz, 2H), 2.97(s, 6H).
Synthetic example 3
[0032] Synthesis of N,N-Dimethyl-3-(p-tolyl)naphthalene-1-amine
[0033]Add 0.20mmol 4-ethynyltoluene, 0.42mmol o-bromoacetophenone, 0.30mmol N,N-dimethylformamide, 0.04mmol cuprous chloride, 0.40mmol sodium hydroxide, and 1.0mL water into the reactor. Under nitrogen atmosphere, heat to 120°C, keep stirring for 30h, stop the reaction, cool to room temperature, add saturated ammonium chloride solution to wash, then extract with ethyl acetate, dry, and distill off the solvent under reduced pressure, the crude product is separated by column chromatography The target product was obtained with a yield of 89%. 1 H NMR (400MHz, CDCl 3 ):δ8.24-8.22(m,1H),7.87-7.85(m,1H),7.70(s,1H),7.62(d,J=8.1Hz,2H),7.49-7.46(m,2H), 7.31-7.29 (m, 3H), 2.96 (s, 6H), 2.43 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com