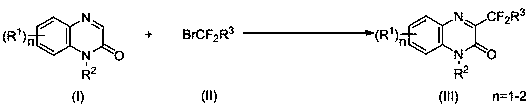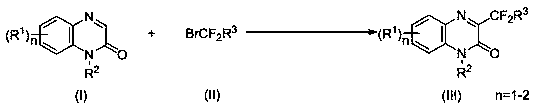Synthesis method of C-3-position difluoromethyl-substituted quinoxalinone derivative
A kind of quinoxalinone and difluoromethyl technology, which is applied in the field of synthesis of C-3-position difluoromethyl substituted quinoxalinone derivatives, can solve problems such as harsh conditions, and achieve reaction safety and reaction selectivity. Good, rich effect of synthetic methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Combine 1-methylquinoxalinone (0.25 mmol, 40 mg), ethyl bromodifluoromethyl acetate (0.5 mmol, 101 mg), Ir(ppy) 3 (1.25 μmol, 0.8 mg), potassium carbonate (0.5 mmol, 69 mg) and N,N -Diisopropylethylamine (0.025mmol, 3.2 mg) was added to a 10 mL solvent storage bottle, and MeCN (1.0 mL) was added as a solvent. 2 Under the protection of the atmosphere, the reaction was carried out for 12 h under 3 w blue light irradiation and 25 ℃. After the reaction, the reaction system was washed with water and extracted with dichloromethane, and then separated into an organic layer and an aqueous layer. After the organic layer was dried with anhydrous sodium sulfate, the solvent was evaporated and concentrated under reduced pressure to obtain a yellow oil. The yellow oil was separated by column chromatography, using a mixture of petroleum ether and ethyl acetate with a volume ratio of 30:1 as the eluent, collecting the eluent containing the target compound, evaporating the solvent and dr...
Embodiment 2
[0027] The inorganic base (potassium carbonate) in the system was replaced with sodium carbonate (0.5 mmol, 52.9 mg), and other operations were the same as in Example 1, to obtain 46 mg of white solid 2,2-difluoro-2-(4-methyl-3) -Oxo-3,4-dihydroquinoxalin-2-yl)-ethyl acetate, the yield is 65%.
Embodiment 3
[0029] will N,N -The dosage of diisopropylethylamine was changed to 0.05 mmol, and the other operations were the same as in Example 1, to obtain 44 mg of white solid 2,2-difluoro-2-(4-methyl-3-oxo-3,4- Dihydroquinoxalin-2-yl)-ethyl acetate, the yield is 62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com