Conductive aluminum lithium ion adsorption column material and preparation method thereof
A conductivity and lithium-ion technology, which is applied in the preparation field of lithium-ion adsorption, can solve the problems that the final product is not cross-linked and solidified, the precursor is not treated with hydrophilicity, and the adsorption effect of the adsorbent is reduced, and the mechanical strength is good. , good acid and alkali resistance, adjustable particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
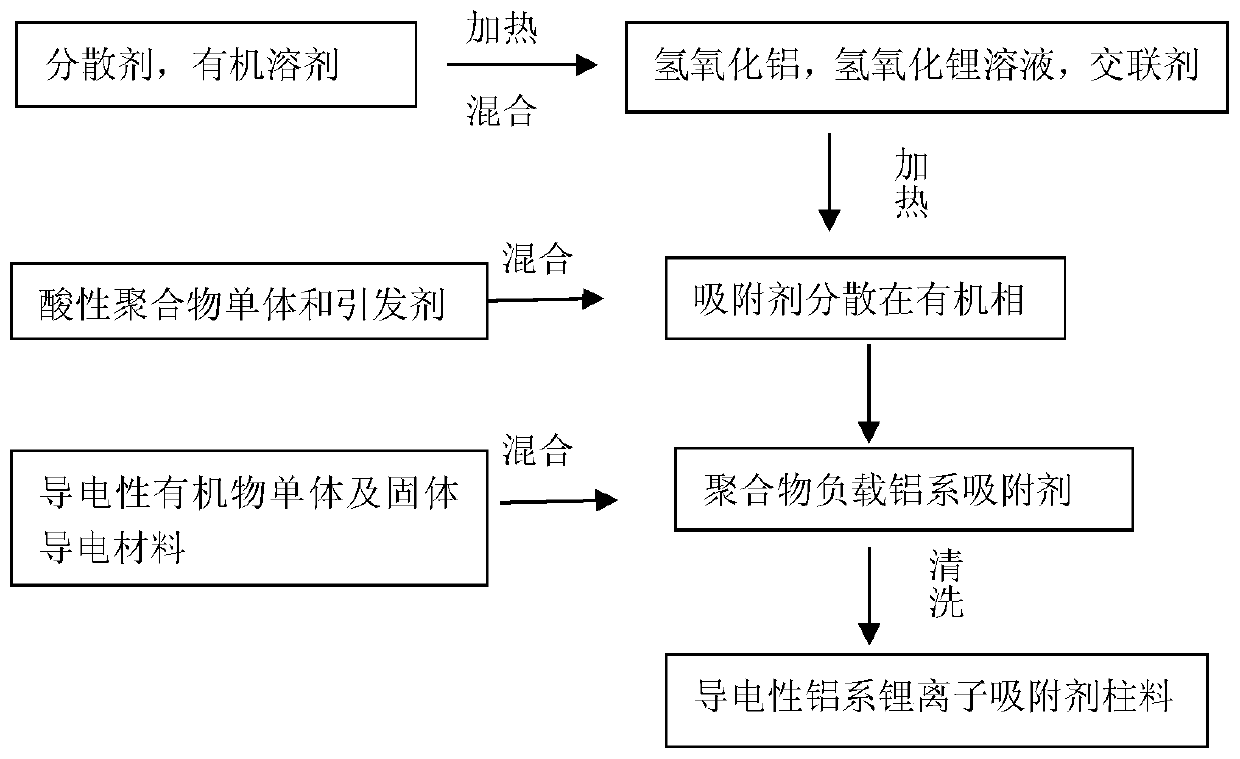
[0024] A method for preparing a conductive aluminum-based lithium ion adsorption column material provided by an aspect of the embodiments of the present invention includes:
[0025] uniformly mix the dispersant and the organic solvent to form the first mixed liquid;
[0026] uniformly mixing the inorganic adsorbent and the crosslinking agent to form a second mixed liquid, the inorganic adsorbent includes aluminum hydroxide and lithium hydroxide;
[0027] After mixing the first mixed solution and the second mixed solution, heat up, then add polymer monomers and initiators to react, and finally add conductive organic monomers and solid conductive materials for polymerization to obtain conductive aluminum-based lithium ion adsorption column material.
[0028]In some embodiments, the preparation method includes: mixing the first mixed liquid with the second mixed liquid, raising the temperature to 55-80° C. and stirring at 50-200 rpm, and condensing and refluxing for 2-6 hours, ...
Embodiment 1
[0060] (1) After mixing 0.5 g of propylene glycol monostearate and 100 g of heptane, they were uniformly stirred to prepare a first mixed liquid.
[0061] (2) 2 g of lithium hydroxide solution, 3 g of secondary water, 10 g of aluminum hydroxide and 0.1 g of diethylenetriamine were mixed, ultrasonically dispersed, and stirred to prepare a second mixed liquid.
[0062] (3) Mix the first mixed liquid with the second mixed liquid, raise the temperature to 55° C., stir at 50 rpm, and reflux for 2 hours.
[0063] (4) Add 5 g of acrylic acid, 0.5 g of ammonium persulfate, and 1.0 g of water. The stirring rate was 300 rpm, and the reaction was carried out for 1 hour.
[0064] (5) Increase the rotation speed to 700 rpm, add 0.1 g of aniline, and 0.1 g of conductive aluminum powder, stir and react for 1 hour, and maintain the temperature at 75°C.
[0065] (6) Stop stirring, filter, and wash with secondary water to obtain a conductive aluminum-based lithium ion adsorption column materi...
Embodiment 2
[0067] (1) After mixing 2.5 g of propylene glycol monostearate and 100 g of heptane, stir evenly to prepare the first mixed liquid.
[0068] (2) 5 g of lithium hydroxide solution, 5 g of secondary water, 25 g of aluminum hydroxide and 0.1 g of diethylenetriamine were mixed, ultrasonically dispersed, and stirred to prepare a second mixed liquid.
[0069] (3) Mix the first mixed liquid with the second mixed liquid, raise the temperature to 80° C., stir at 200 rpm, and reflux for 6 hours.
[0070] (4) Add 25 g of acrylic acid, ammonium persulfate aqueous solution, 3 g of ammonium persulfate, and 5 g of water. The stirring rate was 300 rpm, and the reaction was carried out for 2 hours.
[0071] (5) The rotation speed was increased to 1000 rpm, 2.0 g of aniline and 1.5 g of conductive carbon powder were added, and the reaction was stirred for 5 hours, and the temperature was maintained at 80°C.
[0072] (6) Stop stirring, filter, and wash to obtain a conductive aluminum-based lit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com