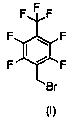
New preparation method of 1-bromo-methyl-2,3,5,6-tetrafluoro-4-(trifluoromethyl)benzene
A technology of bromomethyl and trifluoromethyl, applied in the field of medicinal chemistry, can solve the problems of high price, low yield, and difficult availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
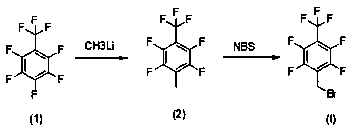
Method used
Image
Examples
Embodiment 1
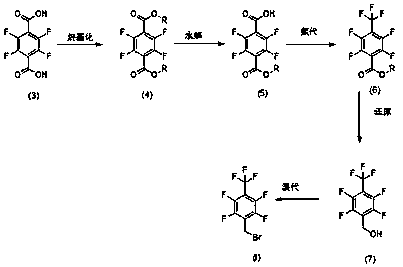
[0056] The first step: Synthesis of 2,3,5,6-tetrafluoro-dimethyl terephthalate [compound of formula (8)]
[0057] The synthetic route is as follows:
[0058]
[0059] Dissolve 15 g of the raw material 2,3,5,6-tetrafluoro-terephthalic acid [compound of formula (3)] in 260 ml of methanol, add 22.9 ml of thionyl chloride dropwise, and keep the internal temperature at 0~10°C, naturally rise to room temperature after dropping, heat to reflux and react overnight. GC detects that the reaction is complete, concentrate the methanol to dryness, add 100ml of water, 100ml of methyl tert-butyl ether, stir and let it stand for liquid separation, wash the water phase twice with 50ml*2 methyl tert-butyl ether, combine the organic phase with 50ml for saturation Wash once with sodium bicarbonate solution, dry over anhydrous sodium sulfate, and concentrate to dryness to obtain 14.5 g of white solid with a yield of 86.51%.
[0060] The NMR detection product is 2,3,5,6-tetrafluoro-dimethyl te...
Embodiment 2
[0084] In the second step, the synthesis of 2,3,5,6-tetrafluoro-4-formic acid methyl ester-benzoic acid [compound of formula (9)], the base used is NaOH, and the rest of the steps are exactly the same as in Example 1.
[0085] The synthetic route is as follows:
[0086]
[0087] Dissolve 8.66 g of the raw material 2,3,5,6-dimethyl phthalate [compound of formula (8)] in 54 ml of methanol, lower the internal temperature to 0°C, and slowly add the prepared NaOH methanol solution dropwise ( 1.24gNaOH+55mlCH 3 OH), after the droplet was completed, it was naturally raised to room temperature, and heated under reflux to react overnight. The HPLC detection reaction is complete, methanol is concentrated to dryness, add 120ml of water, wash with 50ml*3 dichloromethane three times, adjust the pH of the water phase to 1~2, a white solid precipitates, stir for 1h, add 50ml*3 methyl tert-butyl The ether was extracted three times, the organic phase was dried over anhydrous sodium sulfat...
Embodiment 3
[0090] The fourth step is the synthesis of 2,3,5,6-tetrafluoro-4-trifluoromethylbenzyl alcohol [compound of formula (11)], using lithium aluminum hydride as the reducing agent, and the rest of the steps are exactly the same as in Example 1.
[0091] The synthetic route is as follows:
[0092]
[0093] Mix and stir 70g of fluoride compound and 450ml of 2-methyltetrahydrofuran, cool down in an ice-salt bath to below -10°C, add 14.5g of lithium aluminum hydride, keep the reaction for 5 hours, and detect by TLC.
[0094] Pour the reaction solution into 500ml of water and stir, add 200ml of methyl tert-butyl ether for extraction, separate the organic layer, stir and react with 200ml of 1N hydrochloric acid for 20 minutes, remove the acidic aqueous layer, and wash the organic layer with saturated sodium bicarbonate solution to the aqueous layer pH is 7-8. The water layer was separated, the organic layer was dried, and the solvent was distilled off to obtain 68 g of a light yello...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com