Platinum-based compound based on nucleolar stress
A technology of nucleolus stress and compounds, applied in platinum anti-tumor compounds to overcome clinical platinum drug resistance and achieve excellent anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
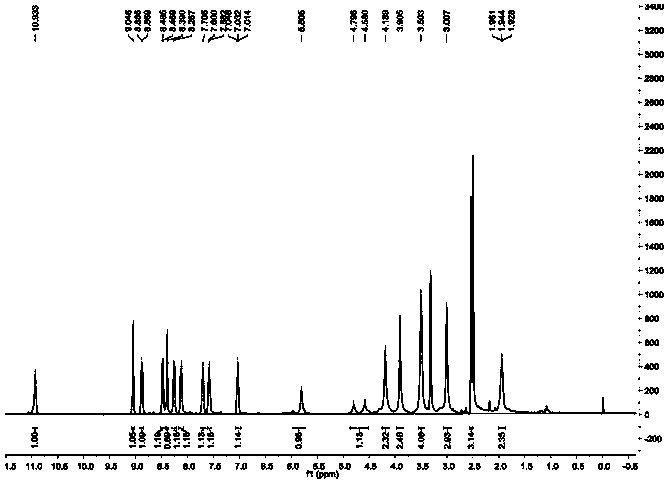
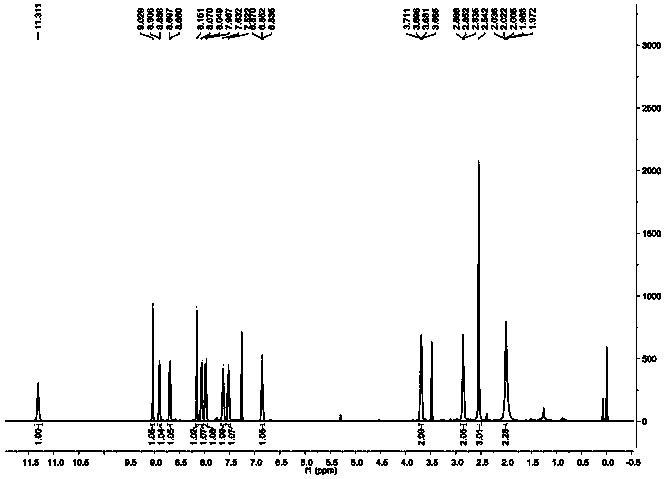
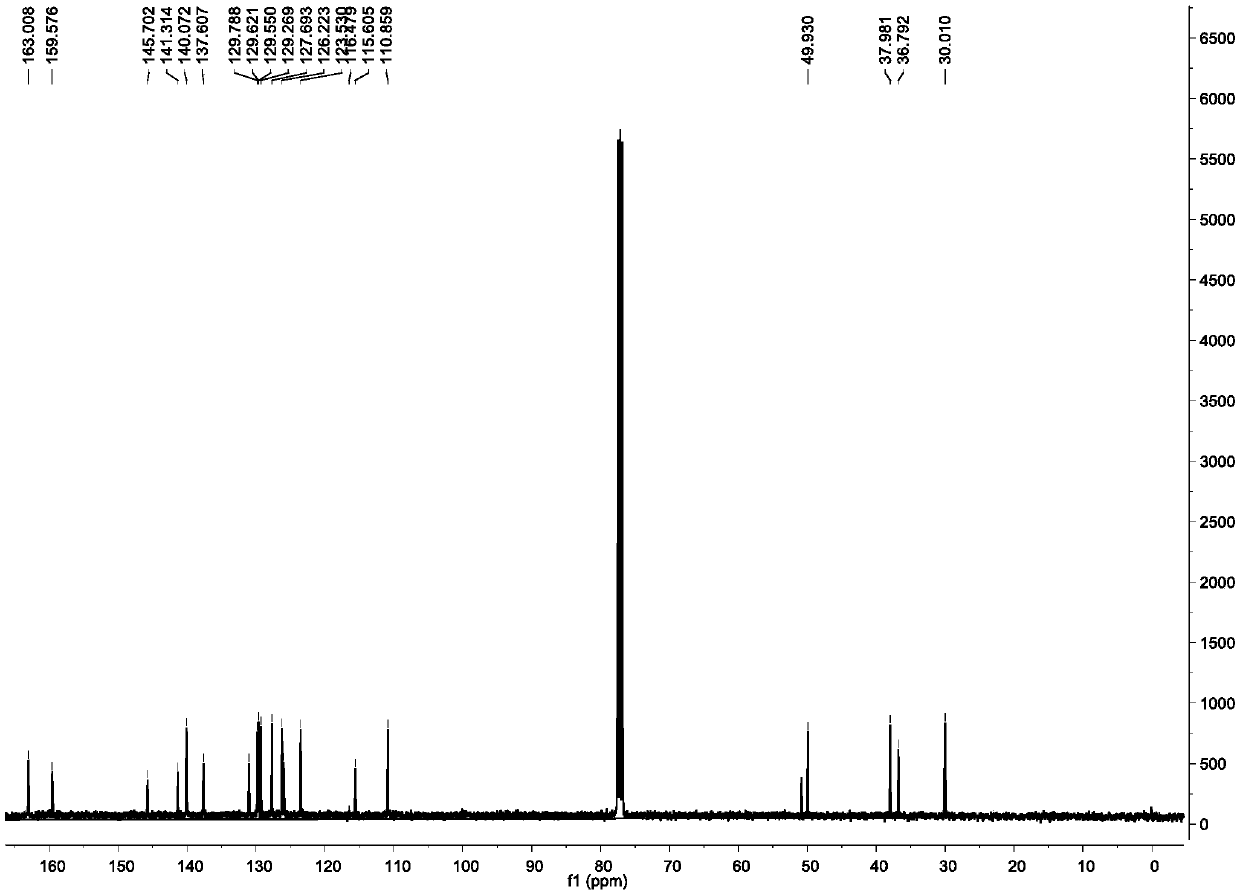
[0043] Synthesis and characterization of a monofunctional platinum-based antitumor compound P1B1 based on nucleolus stress
[0044]
[0045] As shown in the above chemical formula, weigh 1.68g (10.68mmol) 2-chloronicotinic acid (substance 1 in the chemical formula) and 2g (10.68mmol) 3-amino-2-naphthoic acid (substance 2 in the chemical formula) in a round bottom flask, Add 70mL ethanol to dissolve, add 0.9mL (29.13mmol) hydrochloric acid (aqueous hydrogen chloride solution, the concentration is 2mol / L), reflux at room temperature 20-25 degrees Celsius for 72h, monitor the reaction progress by thin-layer chromatography, after the reaction is almost complete, let it stand for cooling . The cooled reaction solution was filtered, and the solid was taken, washed with absolute ethanol, and dried to obtain an orange solid. The obtained orange-yellow solid was uniformly dispersed in ethanol for purification, refluxed at 80°C for 2 hours, suction-filtered, and dried to obtain subs...
Embodiment 2
[0053] Example 2—P1-B1 and cisplatin antitumor activity evaluation
[0054] MTT colorimetry is one of the methods used to detect cell growth and survival in experimental research. In the mitochondria of living cells, exogenous MTT can be reduced by intracellular succinate dehydrogenase to formazan, a purple-blue crystal formazan that is insoluble in water. Formazan is deposited in the cytoplasm of cells, but dead cells do not This phenomenon occurs. When formazan encounters DMSO, the formazan deposited in the cells will be dissolved by DMSO. Use a microplate reader to detect the formazan optical density value (OD value) at a wavelength of 570nm to indirectly reflect the number of living cells. The amount of MTT crystals formed is positively correlated with the number of living cells. Use Graphpad Prism 5 software to process data and calculate IC 50 Value and plot, IC 50 The value refers to the half-inhibitory concentration of the measured drug on cell growth.
[0055] The ...
Embodiment 3
[0060] Based on the copper-catalyzed azido-alkynyl addition reaction (CuAAC reaction), the thymidine analogue 5-ethynyl-2'-deoxyuridine was introduced into the system in order to solve the investigation of DNA replication level, and then The intracellular mechanism of action of the monofunctional platinum compound of the present invention was investigated. 5-Ethynyl-2'-deoxyuridine (EdU) can replace deoxythymine in the newly synthesized DNA duplex during cell proliferation, because it has an alkynyl group that can be labeled with CuAAC reaction, Therefore, fluorescent molecules containing azide modification can be used to selectively label EdU, and effectively detect the level of DNA synthesis in cells and the proportion of cells in S phase. Transcriptional inhibition is an important mechanism of the antitumor effect of platinum-based drugs. Similar to EdU, we introduced 5-ethynyl-2'-uridine nucleoside (EU), based on CuAAC reaction, and used AlexaFluor 647- Azide dye labeling...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com