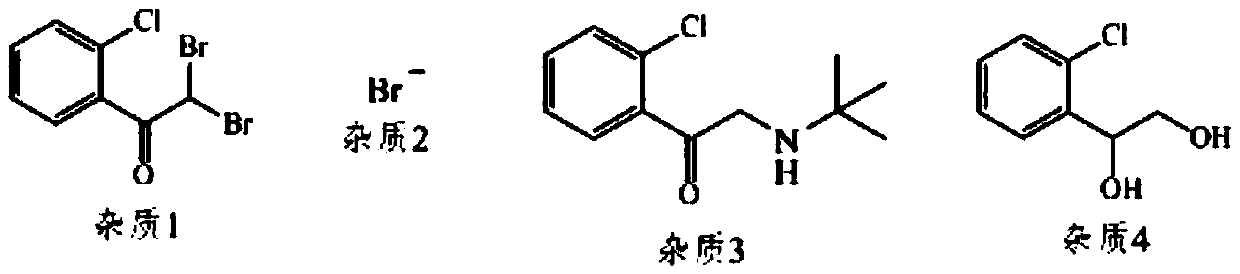
Method for industrial production of tulobuterol
A technology of tulobuterol and oxidant, applied in the field of drug synthesis, can solve the problems of difficult removal of toxic impurities and high content of impurities, and achieve the effects of reduced production costs, low cost of raw materials, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
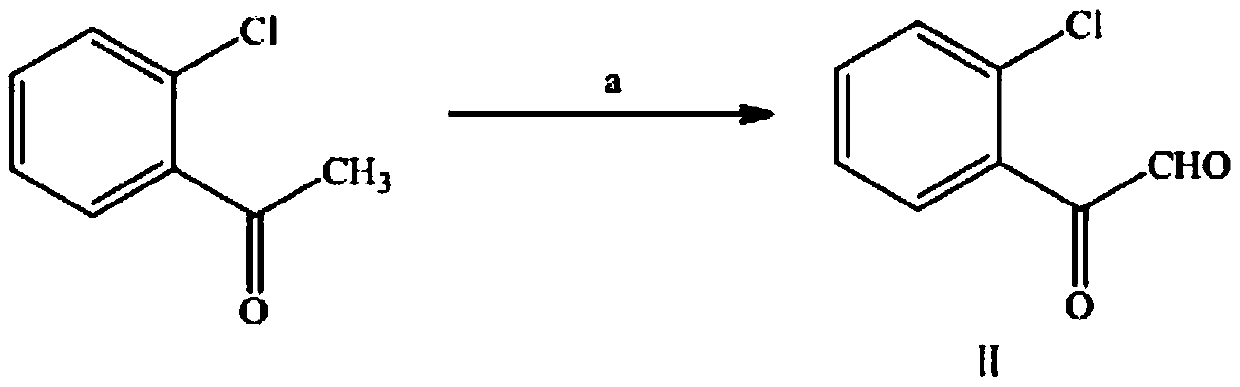
[0029] 1. Preparation of Intermediate II
[0030] Put 15.4g (0.1mol) of o-chloroacetophenone, 20mL of DMSO and 6g of iodine into a three-necked flask, raise the temperature to 55-60°C and stir for 1-1.5h, and identify the end point of the reaction by TLC (developing agent: ethyl acetate- Petroleum ether = 1:1), after the reaction was completed, cooled to room temperature, added 5 mL of deionized water, added 10 mL of 10% sodium thiosulfate solution, stirred for 10 min, extracted with isopropyl acetate, washed the organic layer twice with water, and saturated salt Washed twice with water, dried over anhydrous sodium sulfate, filtered to remove the desiccant, and concentrated under reduced pressure to obtain 14.5 g (0.086 mol) of intermediate II with a yield of 86.1%.
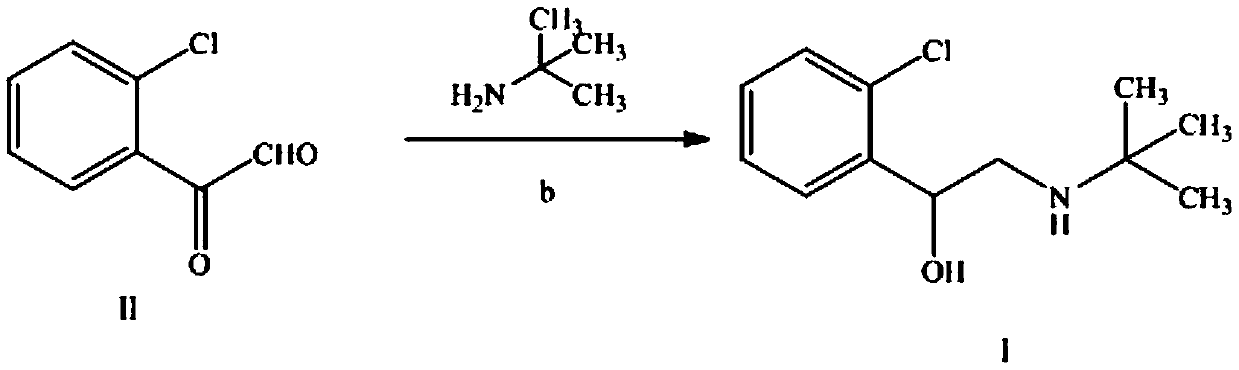
[0031] Two, the preparation of compound Ⅰ (tulobuterol crude product)
[0032] Add 14g (0.083mol) of intermediate II, 30mL methanol, 6.6g (0.09mol) tert-butylamine, and 4g sodium borohydride to a dry three-necke...
Embodiment 2
[0036] 1. Preparation of Intermediate II
[0037] Put 154g (1mol) of o-chloroacetophenone, 200mL of DMSO and 60g of iodine into a three-necked flask, raise the temperature to 55-60°C and stir for 1-1.5h, and identify the end point of the reaction by TLC (developing solvent: ethyl acetate-petroleum ether =1:1), after the reaction is completed, cool to room temperature, add 50 mL of deionized water, add 100 mL of 10% sodium thiosulfate solution, stir for 10 min, extract with isopropyl acetate, wash the organic layer twice with water, and wash with saturated saline Twice, dried over anhydrous sodium sulfate, filtered to remove the desiccant, and concentrated under reduced pressure to obtain 153 g (0.91 mol) of intermediate II with a yield of 91.1%.
[0038] Two, the preparation of compound Ⅰ (tulobuterol crude product)
[0039] Add 150g (0.893mol) of intermediate II, 330mL methanol, 6g (0.9mol) tert-butylamine, and 43g sodium borohydride into a dry three-necked flask in sequence...
Embodiment 3
[0043] 1. Preparation of Intermediate II
[0044] Put 154g (1mol) of o-chloroacetophenone, 200mL of DMSO and 60g of iodine into a three-necked flask, raise the temperature to 55-60°C and stir for 1-1.5h, and identify the end point of the reaction by TLC (developing solvent: ethyl acetate-petroleum ether =1:1), after the reaction is completed, cool to room temperature, add 50 mL of deionized water, add 100 mL of 10% sodium thiosulfate solution, stir for 10 min, extract with isopropyl acetate, wash the organic layer twice with water, and wash with saturated saline Twice, dried over anhydrous sodium sulfate, filtered to remove the desiccant, and concentrated under reduced pressure to obtain 147 g (0.88 mol) of intermediate II with a yield of 87.5%.
[0045] Two, the preparation of compound Ⅰ (tulobuterol crude product)
[0046] Add 150g (0.893mol) of intermediate II, 350mL ethanol, 6g (0.9mol) tert-butylamine, and 43g sodium borohydride into a dry three-necked flask in turn, con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com