Isatin compound and its preparation method and application
A compound, the technology of isatin, which is applied in the field of isatin compounds and its preparation, can solve the problems of limited medicine, large neurotoxicity, poor water solubility and high cytotoxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Compound preparation
[0045] The synthetic route of compound sees following reaction formula:
[0046]
[0047] Reaction conditions: (a) potassium carbonate, DMF, room temperature; (b) sodium carbonate; tetrakis(triphenylphosphine)palladium; 1,4-dioxane and water; 95°C; (c) cesium carbonate, DMSO, 100℃; (d) palladium carbon / H 2 ; methanol, room temperature; (e) pyridine, DCM, 0 °C to room temperature.
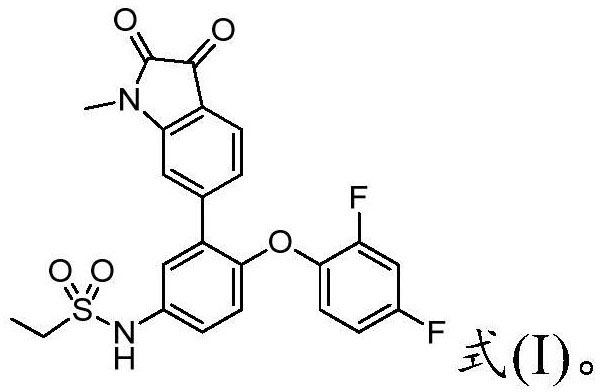
[0048] In compound 5, R is selected from ethyl, propyl, cyclopropyl, thienyl, substituted and unsubstituted phenyl; further, the compound 5 includes the following structure: wherein YHP-1 is the compound of formula (I).
[0049]
[0050] Concrete reaction process is as follows:
[0051] (1) Preparation of intermediate 6-bromo-1-methylindoline-2,3-dione (1)
[0052] Dissolve 1.15g (5mmol) 6-bromoisatin in 10mL DMF, add 1.03g (7.5mmol) potassium carbonate under stirring, then add 0.804g (6.25mmol) dimethyl sulfate dropwise, react at room temperature ...
Embodiment 2
[0081] Example 2 Detection of inhibitory activity of compounds on BRD4 protein
[0082] The inhibitory activity of compounds on BRD4 was tested by Homogeneous Time-Resolved Fluorescence (HTRF). The specific method is as follows:
[0083] Experimental principle: HTRF combines the advantages of fluorescence resonance energy transfer FRET and time-resolved fluorescence TRF, and integrates the homogeneous experimental method of FRET and the low background characteristics of TRF. It has the advantages of simple operation, high sensitivity and large test throughput. , The experimental data is stable and reliable.
[0084] (1) Compounds were diluted with DMSO.
[0085] (2) Use the Diluent Buffer in the kit to dilute BRD4 (BD2, BD2) and Biotin-labeled histone H4 peptides, and prepare the reaction solution.
[0086] (3) Use Dtection Buffer in the kit to dilute Anti-GST-TB 2+ Cryptate and SA-XL-665, and configure the detection solution.
[0087] (4) Take a 384-well plate and arra...
Embodiment 3
[0094] Example 3: EC of YHP-1 in J-Lat HIV-1 latent infection cell line 50
[0095] Experiment principle: EC 50 is the half-maximum effect concentration, which refers to the concentration of the compound when the compound reaches the 50% maximum effect of activating HIV latent activity, and the unit is μM.
[0096] Experimental procedure: take well-grown J-Lat cells and spread them in 96-well transparent plates, and the amount of cells used is 2×10 per well. 5 Add different concentrations of the test compound respectively, the final concentrations are 320, 160, 80, 40, 20, 10, 5, 0 μM respectively, JQ1 is used as the positive control, the untreated group is the negative control, and each concentration is at least 3 Duplicate wells, each experiment was repeated 3 times. After culturing in a 5% CO2 incubator for 24 hours, the cells were collected by centrifugation, the supernatant was discarded, washed once with PBS, the supernatant was discarded, and then resuspended with PB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com