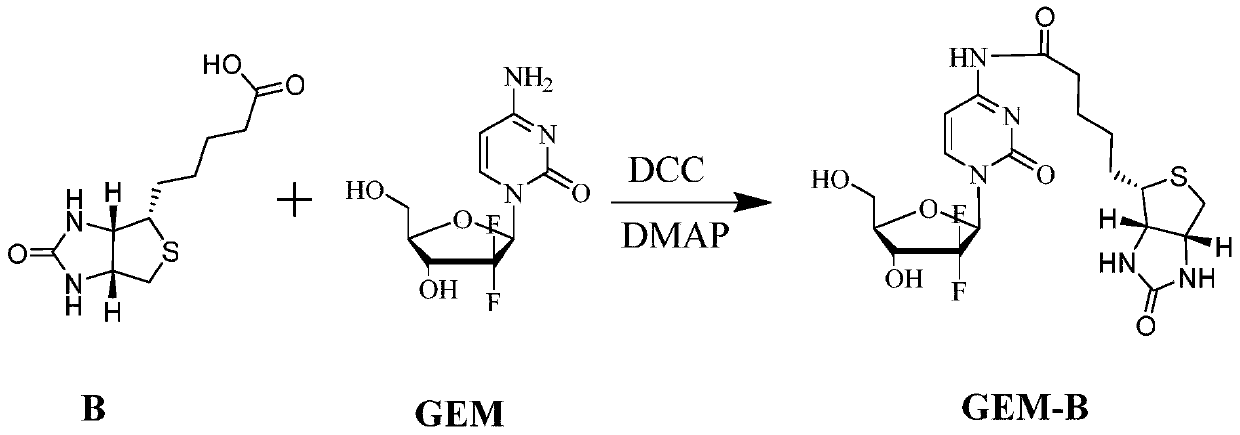
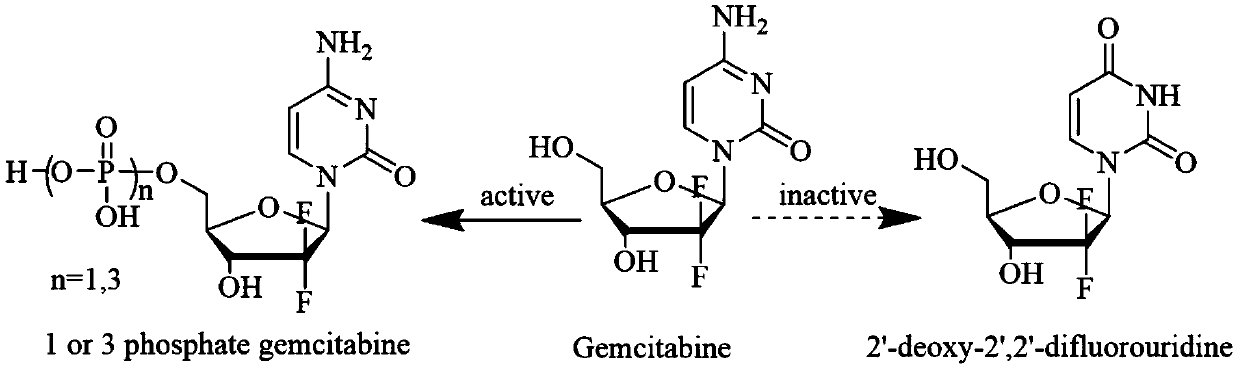
Gemcitabine prodrug with tumor targeting properties and preparation method and application thereof
A tumor-targeted, gemcitabine technology, which can be used in anti-tumor drugs, preparation of sugar derivatives, medical preparations containing active ingredients, etc. Selectivity, excellent tumor targeting selectivity, and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of Biotin-Gemcitabine Prodrug
[0040] Dissolve biotin (0.18 mmol) in 60 ml of DMSO, stir at 0°C for 6 h under the action of dehydrating agent DCC (4.0 mmol) and catalyst DMAP (4.0 mmol); then add gemcitabine dissolved in DMSO dropwise, biotin and The molar ratio of gemcitabine is 1:3; raise the temperature from the ice bath to room temperature 25°C, and stir overnight in the dark; filter to remove by-products, concentrate the filtrate, recrystallize from ice ether or isopropanol, and purify by chromatography or preparative liquid phase , lyophilized to obtain tumor-targeted gemcitabine prodrug GEM-B, C 19 H 25 F 2 N 5 O 6 S, the theoretical MW is 489.5 (the yield is about 70%), the ion peak MS is identified by mass spectrometry + is 490.6. The structural characterization data is as follows, and its structure is determined as shown in formula (I):
[0041] 1 H-NMR (300MHz, DMSO): δ1.25-1.28 (m, CH 2 ,2H), 1.55-1.60(m, 2CH 2 ,4H), 2.35-2....
Embodiment 2
[0042] Example 2: Preparation of Biotin-Gemcitabine Prodrug
[0043] 1) Preparation of biotin active ester (B-NHS): take biotin (0.62 g, 2.5 mmol) and dissolve it in 40 ml of anhydrous DMF, add 0.5 ml of TEA triethylamine and mix and stir at room temperature in an anhydrous environment in the dark Uniform; then mixed 0.51g (2.5mmol) of DCC and 0.28g (2.5mmol) of NHS, stirred in the dark for 24h, filtered to remove by-product dicyclohexylurea, dried under low temperature vacuum to remove DMF and TEA, and the product was precipitated with ether to obtain biotin Active ester B-NHS.
[0044] 2) Preparation of biotin-gemcitabine prodrug: take biotin active ester B-NHS (0.15mmol) and dissolve it in 50ml of anhydrous DMSO / TEA (volume ratio 2:1), take an equimolar amount of B-NHS. The gemcitabine was added to the mixed solution and reacted overnight under anhydrous conditions in the dark. The reaction solution was vacuum-dried, recrystallized with glacial ether or isopropanol, purifi...
Embodiment 3
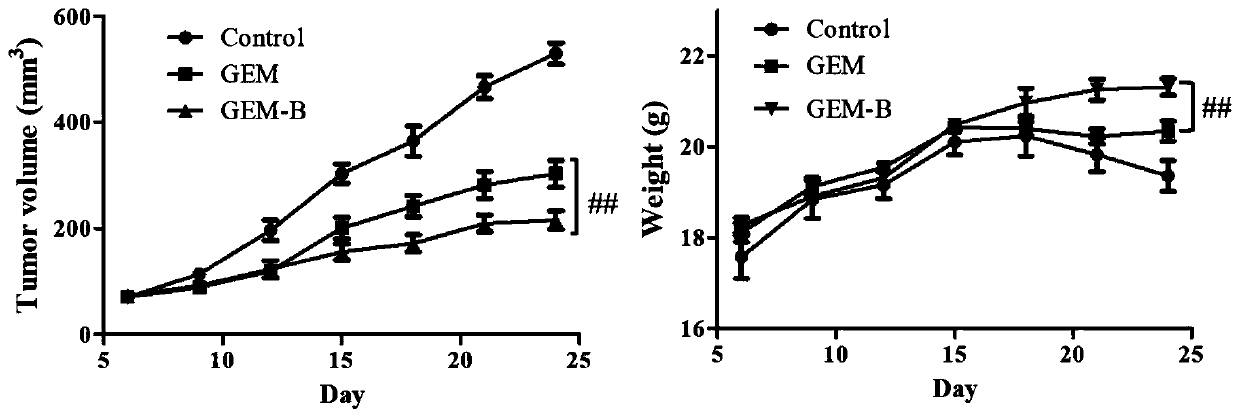
[0046] Example 3: Cytotoxicity detection and evaluation experiment
[0047] Comparison of in vitro anticancer effect evaluation of GEM and GEM-B. In view of the fact that gemcitabine nucleoside drugs are broad-spectrum anti-cancer cell poisons, a variety of cancer tissue-derived biotin receptor-positive tumor cells (HeLa, HepG2, BXPC-3, MDA-MB-231 and SK) were used in this example. -OV-3) Evaluate the efficacy of the corresponding conjugated product GEM-BIOTIN prepared in Example 2 and its prototype compound, and conduct the LO2 liver cell toxicity test to normal cells.
[0048] Take the cells in the logarithmic growth phase and inoculate 2-10×10 cells according to the size of the cells 3 After 24 hours of growth, the supernatant was discarded, and then the drugs were divided into groups as follows: cancer cells were divided into a drug-free group and a drug-added group (concentration 0.05-50 μM for cancer cells, 0.5-100 μM for LO2 cells) ; wherein the biotin concentration i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com