Ferronickel catalytic material and preparation method thereof, and application of ferronickel catalytic material in preparing hydrogen by electrolyzing water and preparation of liquid solar fuel
A catalytic material, a technology for electrolyzing water, applied in catalyst activation/preparation, chemical instruments and methods, hydroxyl compound preparation, etc., can solve the problems of high difficulty, complex process, high energy consumption, avoid unsafe factors and improve yield , The effect of reducing the energy consumption per unit of hydrogen production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
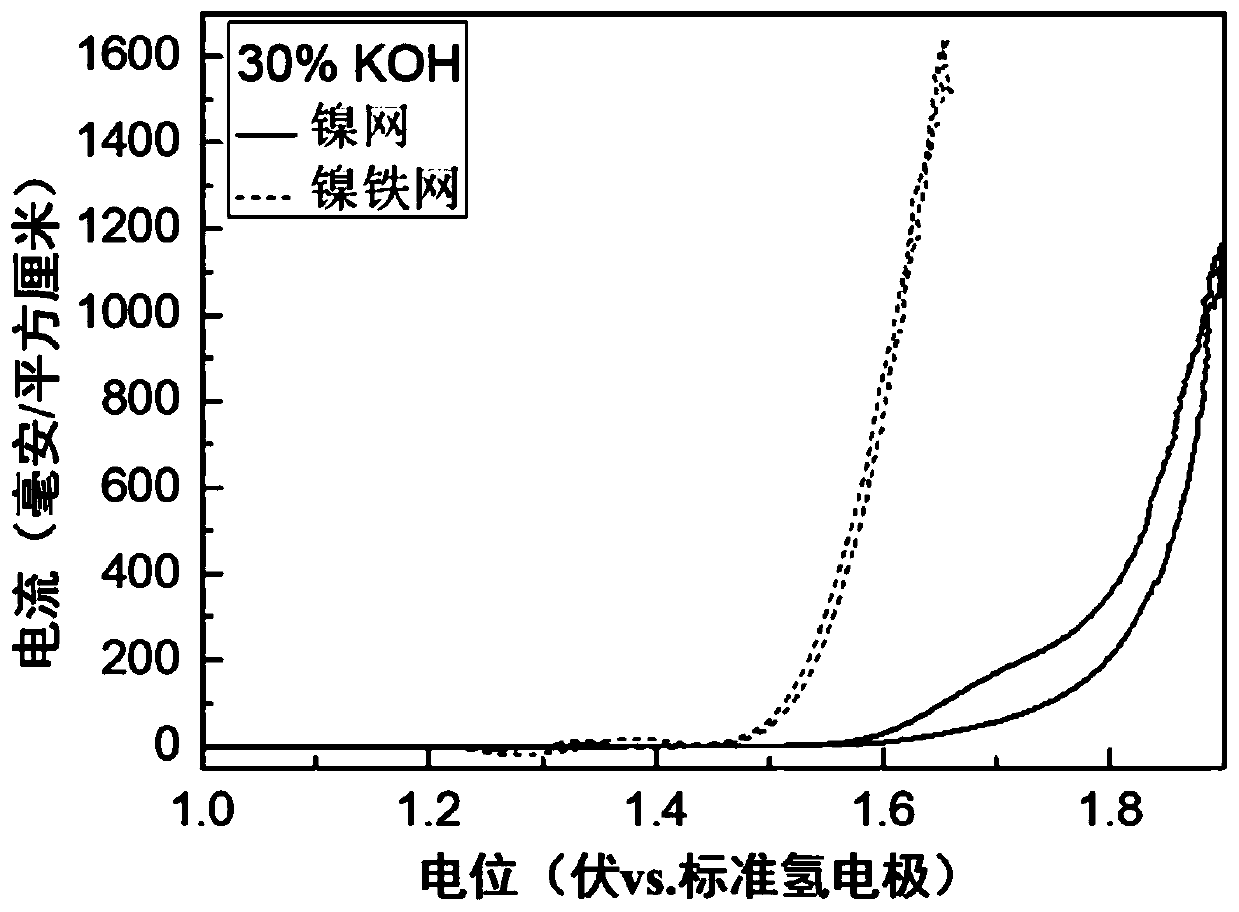
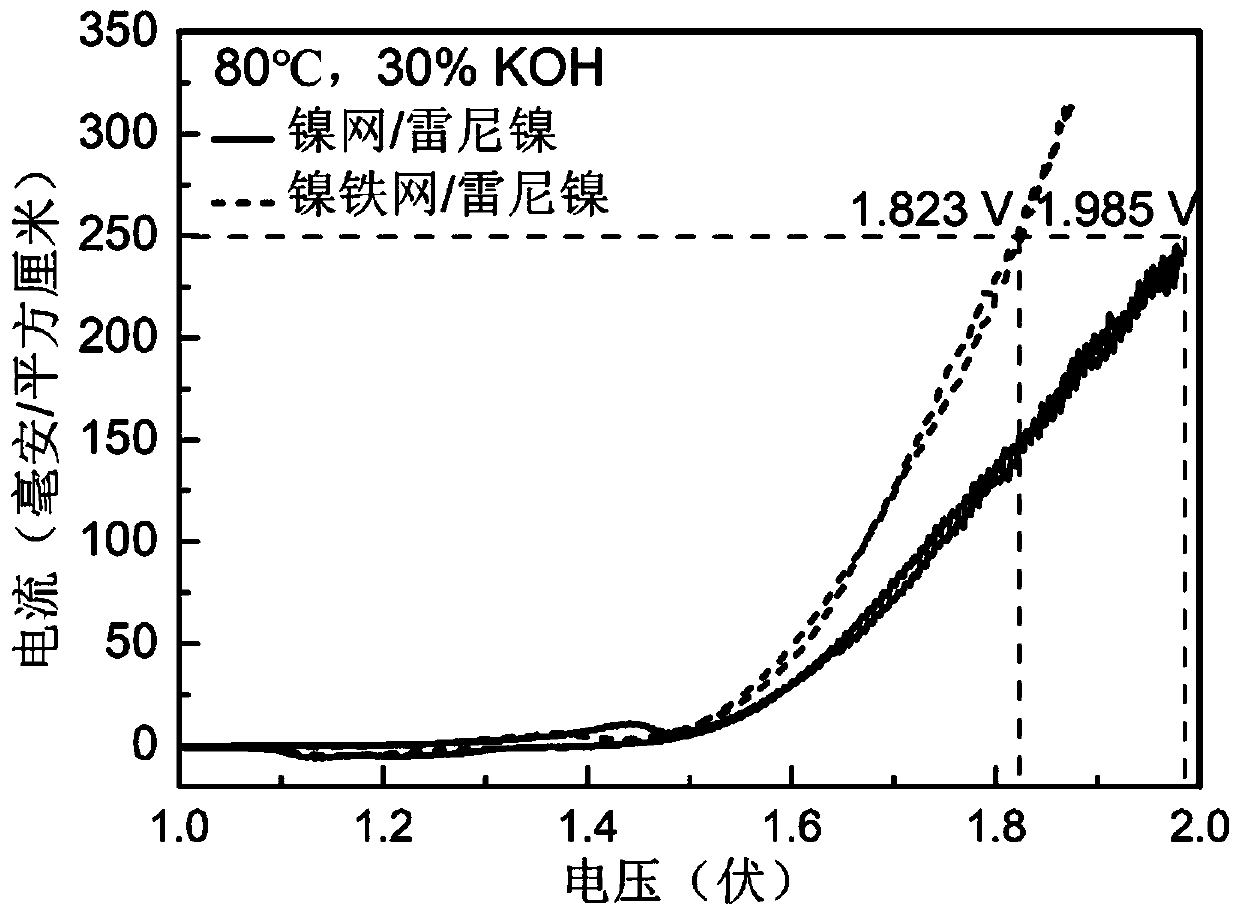
[0088] A new nickel-iron anode catalyst was prepared on the basis of commercially available nickel mesh, and commercially available Raney nickel cathode was used to electrolyze water under the condition of strong alkali and high temperature (30%KOH, 80℃).
[0089] (1) A 60-mesh Raney nickel cathode catalyst is used in commercial alkaline electrolyzed water industrial equipment, soaked in 1M NaOH solution for 24 hours, rinsed with deionized water until the solution is neutral, and dried at room temperature.
[0090] (2) Preparation of nickel-iron mesh anode catalyst.
[0091] (2-1) A 60-mesh nickel mesh is used in the commercial alkaline electrolyzed water industrial equipment and placed in a tube furnace. In a stable atmosphere with a hydrogen:argon volume ratio of 1:10, it is kept at 400°C for 5 hours. After cooling to room temperature, a nickel mesh substrate with specific surface species is obtained;
[0092] (2-2) Prepare a reaction solution. Ferric chloride is dissolved i...
Embodiment 2
[0101] The metal nickel substrate was not modified by surface species, and the iron element was directly introduced to prepare the nickel-iron catalyst as a control example.
[0102] (1) Preparation of nickel-iron mesh anode catalyst.
[0103] (1-1) Prepare a reaction solution. Ferric chloride is dissolved in water and is configured as a transparent solution of 10 mmol / liter;
[0104] (1-2) Low temperature chemical bath. Immerse the 60-mesh nickel mesh used in the commercially available alkaline electrolyzed water industrial equipment in the stable reaction solution in step (1-1), and let the whole system stand at 50 degrees for 6 hours;
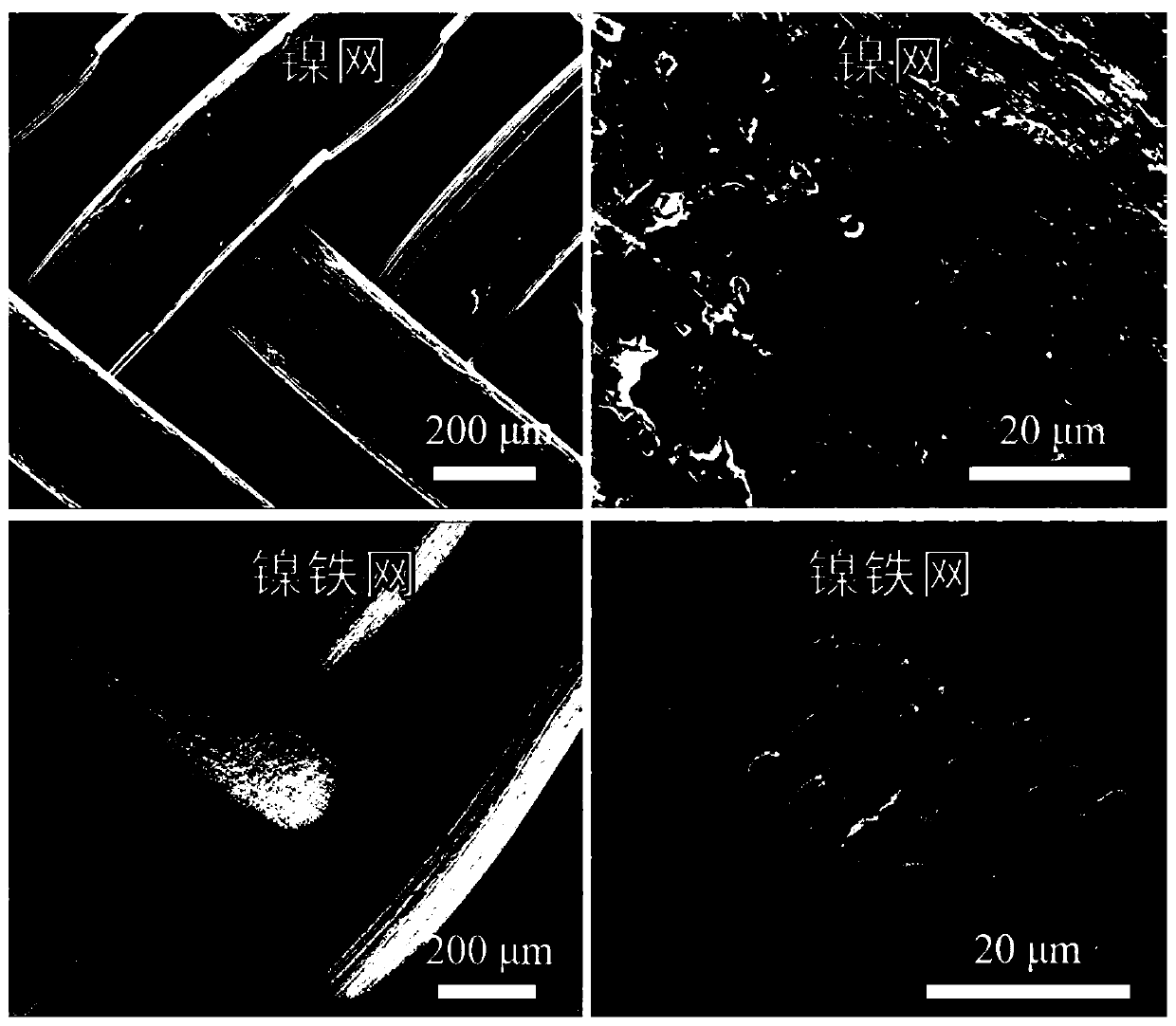
[0105] (1-3) Rinse the electrodes. Take out the nickel mesh after standing in (1-2), and fully wash it with a large amount of water, dry the electrode piece or dry it under natural conditions to obtain a nickel-iron mesh catalyst, which is designated as sample 2 # . Sample 2 was examined with a scanning electron microscope # Morphologi...
Embodiment 3
[0109] When iron was introduced to prepare the nickel-iron catalyst, the reaction temperature increased, as a comparative example.
[0110] (1) Preparation of nickel-iron mesh anode catalyst.
[0111] (1-1) A 60-mesh nickel mesh is used in the commercially available alkaline electrolyzed water industrial equipment, placed in a tube furnace, and kept at 400°C for 5 hours in a stable atmosphere with a hydrogen:argon volume ratio of 1:10. After cooling to room temperature, a nickel mesh substrate with specific surface species is obtained;
[0112] (1-2) Prepare a reaction solution. Ferric chloride is dissolved in water and is configured as a transparent solution of 10 mmol / liter;
[0113] (1-3) Low temperature chemical bath. Immerse the 60-mesh nickel mesh used in the commercially available alkaline electrolyzed water industrial equipment in the stable reaction solution in step (1-2), and let the whole system stand at 85 degrees for 6 hours;
[0114] (1-4) Rinse the electrode...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com