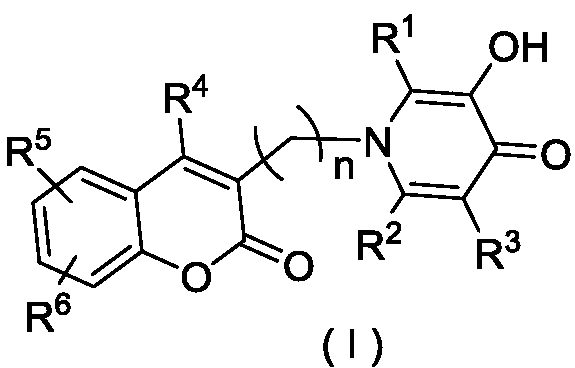
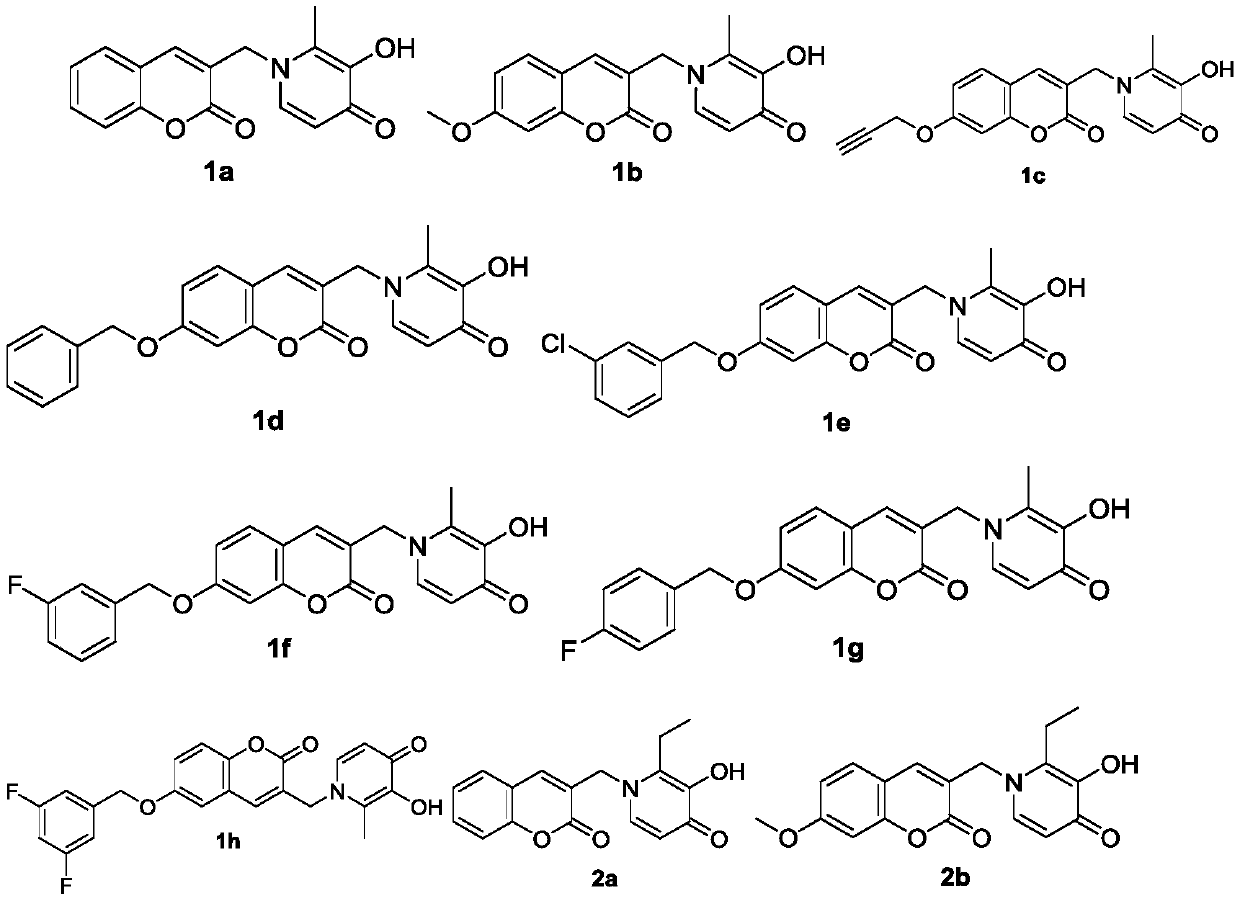
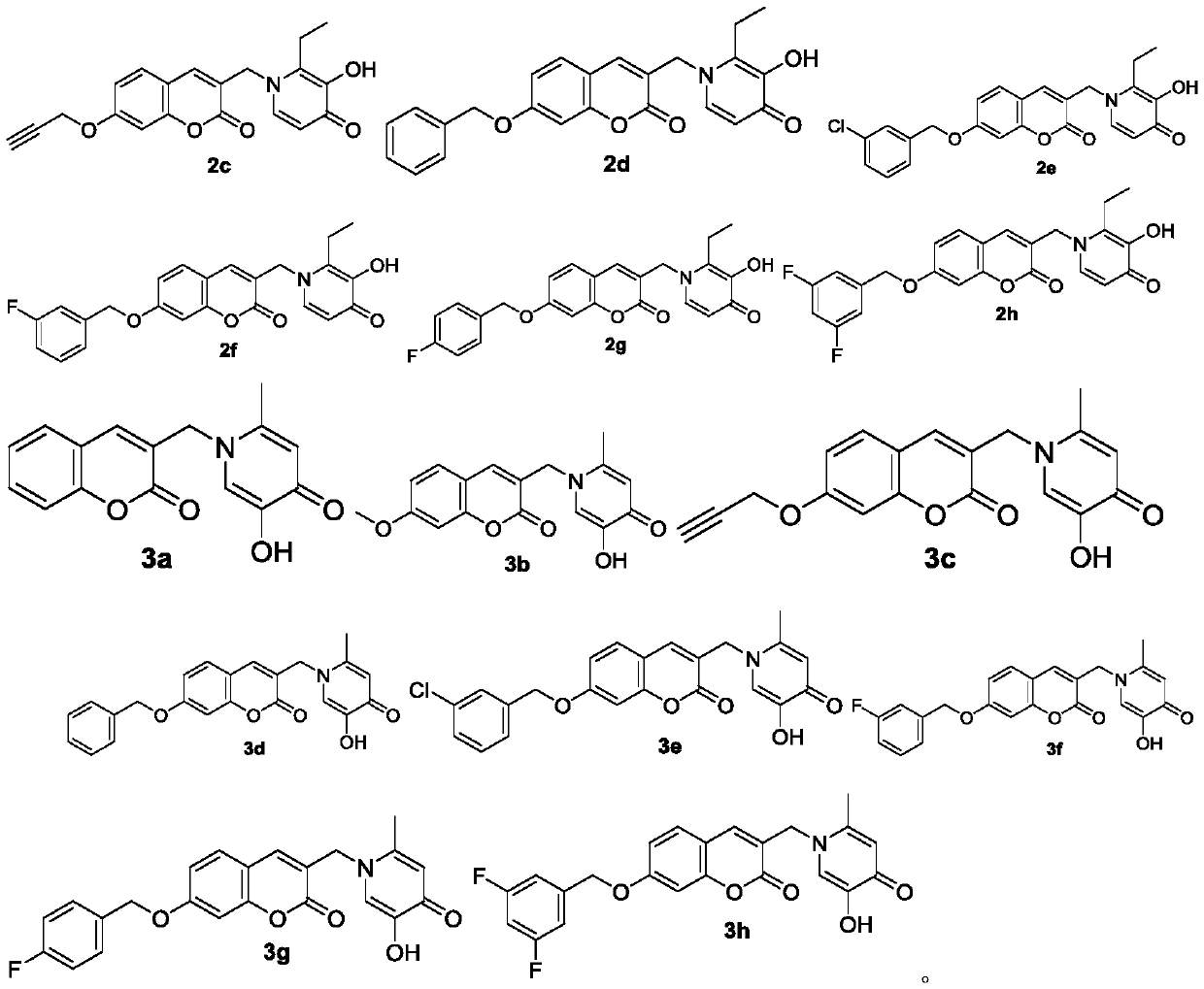
Coumarin heterozygous pyridone compounds having iron chelation and monoamine oxidase B inhibitory activity as well as preparation and application of compounds
A technology of pyridone and coumarin, applied in the direction of organic active ingredients, organic chemistry, medical preparations containing active ingredients, etc., can solve the problem of increasing neurotransmitters in the brain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 2-Methyl-3-hydroxy-1-((coumarin-3-yl)methyl)pyridin-4(1H)-one (1a)
[0059] Add maltol (7.56g, 60mmol), acetone 100mL, and methyl iodide (9.37g, 66mmol) into a 250mL single-necked bottle, heat and reflux for 6 hours, cool to room temperature after the reaction, spin to dry the solvent, add 100mL water to dissolve, and Methyl chloride (50 mL) was extracted 4 times, the organic layer was dried over anhydrous sodium sulfate, and the organic layer was concentrated to obtain 2-methyl-3-methoxypyrone. Yield 98%.
[0060] Add the pyrone (8.65g, 40mmol) obtained by the above reaction into a 250mL one-mouth bottle, 60mL of 25% ammonia water, 50mL of ethanol, heat and reflux at 75°C for 12h, cool to room temperature after the reaction is completed, spin the solvent to obtain a brown oily liquid, and use Acetone / ethyl acetate recrystallization gave 2-methyl-3-methoxypyridone as a light yellow solid. Yield 75%.
[0061] Add salicylaldehyde (7.33g, 60mmol), propionic anhydride (3...
Embodiment 2
[0070] The preparation method of 2-methyl-3-hydroxyl-1-((7-methoxy-coumarin-3-yl)methyl)pyridin-4(1H)-one (1b)
[0071]
[0072]Add maltol (7.56g, 60mmol), acetone 100mL, and methyl iodide (9.37g, 66mmol) into a 250mL single-necked bottle, heat and reflux for 6 hours, cool to room temperature after the reaction, spin to dry the solvent, add 100mL water to dissolve, and Methyl chloride (50 mL) was extracted 4 times, the organic layer was dried over anhydrous sodium sulfate, and the organic layer was concentrated to obtain 2-methyl-3-methoxypyrone. Yield 98%.
[0073] Add the pyrone (8.65g, 40mmol) obtained by the above reaction into a 250mL one-mouth bottle, 60mL of 25% ammonia water, 50mL of ethanol, heat and reflux at 75°C for 12h, cool to room temperature after the reaction is completed, spin the solvent to obtain a brown oily liquid, and use Acetone / ethyl acetate recrystallization gave 2-methyl-3-methoxypyridone as a light yellow solid. Yield 75%.
[0074] Add 2-hydro...
Embodiment 3
[0082] The preparation method of 2-methyl-3-hydroxyl-1-((7-propargyloxy-coumarin-3-yl)methyl)pyridin-4(1H)-one (1c)
[0083]
[0084] Add maltol (7.56g, 60mmol), acetone 100mL, and benzyl bromide (11.29g, 66mmol) into a 250mL single-necked bottle, heat and reflux for 6 hours, cool to room temperature after the reaction, spin the solvent, add 100mL water to dissolve, and Methyl chloride (50 mL) was extracted 4 times, the organic layer was dried over anhydrous sodium sulfate, and the organic layer was concentrated to obtain 2-methyl-3-benzyloxypyrone. Yield 98%.
[0085] Add the pyrone (8.65g, 40mmol) obtained by the above reaction into a 250mL one-mouth bottle, 60mL of 25% ammonia water, 50mL of ethanol, heat and reflux at 75°C for 12h, cool to room temperature after the reaction is completed, spin the solvent to obtain a brown oily liquid, and use Acetone / ethyl acetate recrystallization gave 2-methyl-3-benzyloxypyridone as a light yellow solid. Yield 75%.
[0086] Add 2,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com