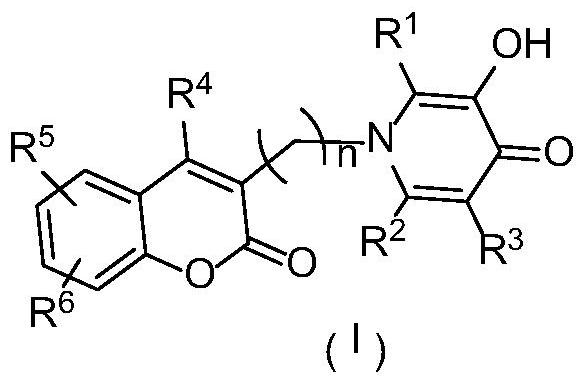
Coumarin hybrid pyridone compound with iron chelation and monoamine oxidase b inhibitory activity and its preparation and application
A pyridone, coumarin technology, applied in the direction of organic active ingredients, organic chemistry, medical preparations containing active ingredients, etc., can solve problems such as increasing neurotransmitters in the brain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
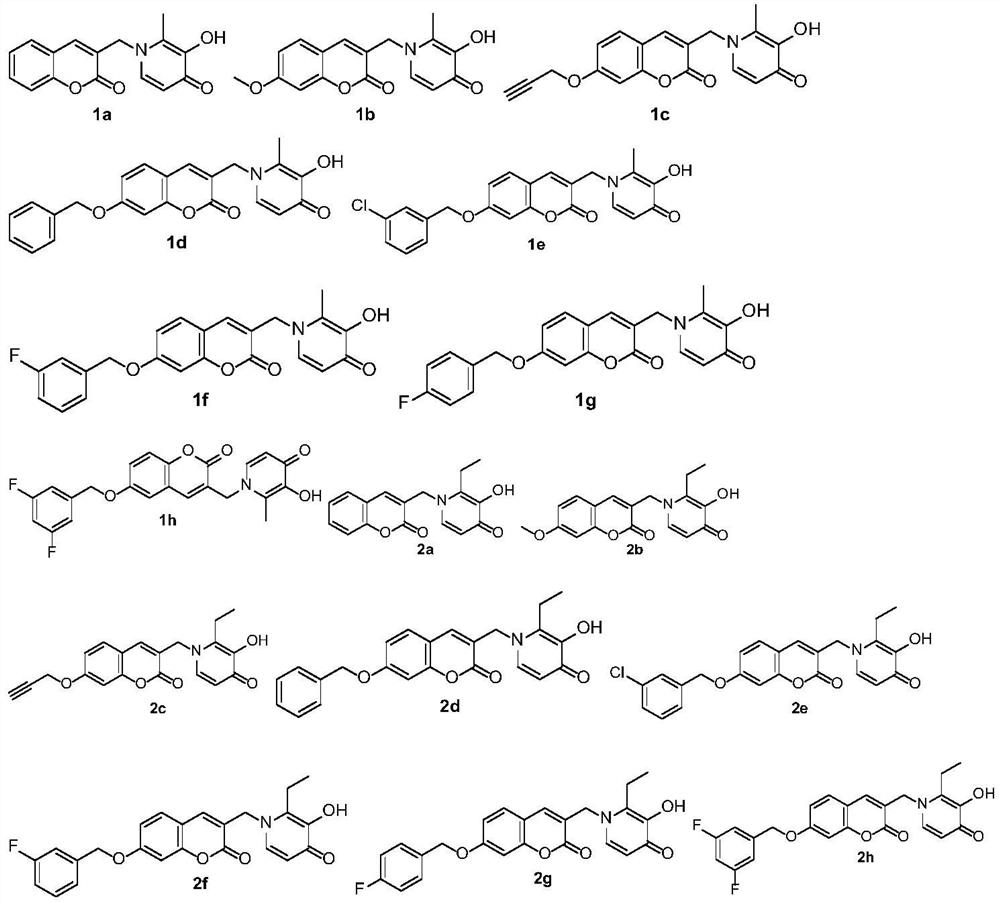
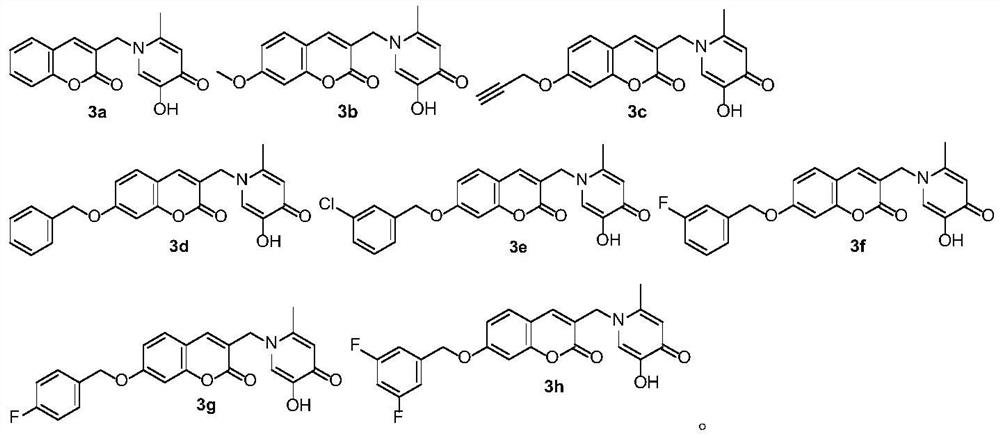
[0058] 2-Methyl-3-hydroxy-1-((coumarin-3-yl)methyl)pyridin-4(1H)-one (1a)
[0059] Add maltol (7.56g, 60mmol), acetone 100mL, and methyl iodide (9.37g, 66mmol) into a 250mL single-necked bottle, heat and reflux for 6 hours, cool to room temperature after the reaction, spin to dry the solvent, add 100mL water to dissolve, and Methyl chloride (50 mL) was extracted 4 times, the organic layer was dried over anhydrous sodium sulfate, and the organic layer was concentrated to obtain 2-methyl-3-methoxypyrone. Yield 98%.
[0060] Add the pyrone (8.65g, 40mmol) obtained by the above reaction into a 250mL one-mouth bottle, 60mL of 25% ammonia water, 50mL of ethanol, heat and reflux at 75°C for 12h, cool to room temperature after the reaction is completed, spin the solvent to obtain a brown oily liquid, and use Acetone / ethyl acetate recrystallization gave 2-methyl-3-methoxypyridone as a light yellow solid. Yield 75%.
[0061] Add salicylaldehyde (7.33g, 60mmol), propionic anhydride (3...
Embodiment 2
[0070] The preparation method of 2-methyl-3-hydroxyl-1-((7-methoxy-coumarin-3-yl)methyl)pyridin-4(1H)-one (1b)
[0071]
[0072]Add maltol (7.56g, 60mmol), acetone 100mL, and methyl iodide (9.37g, 66mmol) into a 250mL single-necked bottle, heat and reflux for 6 hours, cool to room temperature after the reaction, spin to dry the solvent, add 100mL water to dissolve, and Methyl chloride (50 mL) was extracted 4 times, the organic layer was dried over anhydrous sodium sulfate, and the organic layer was concentrated to obtain 2-methyl-3-methoxypyrone. Yield 98%.
[0073] Add the pyrone (8.65g, 40mmol) obtained by the above reaction into a 250mL one-mouth bottle, 60mL of 25% ammonia water, 50mL of ethanol, heat and reflux at 75°C for 12h, cool to room temperature after the reaction is completed, spin the solvent to obtain a brown oily liquid, and use Acetone / ethyl acetate recrystallization gave 2-methyl-3-methoxypyridone as a light yellow solid. Yield 75%.
[0074] Add 2-hydro...
Embodiment 3
[0082] The preparation method of 2-methyl-3-hydroxyl-1-((7-propargyloxy-coumarin-3-yl)methyl)pyridin-4(1H)-one (1c)
[0083]
[0084] Add maltol (7.56g, 60mmol), acetone 100mL, and benzyl bromide (11.29g, 66mmol) into a 250mL single-necked bottle, heat and reflux for 6 hours, cool to room temperature after the reaction, spin the solvent, add 100mL water to dissolve, and Methyl chloride (50 mL) was extracted 4 times, the organic layer was dried over anhydrous sodium sulfate, and the organic layer was concentrated to obtain 2-methyl-3-benzyloxypyrone. Yield 98%.
[0085] Add the pyrone (8.65g, 40mmol) obtained by the above reaction into a 250mL one-mouth bottle, 60mL of 25% ammonia water, 50mL of ethanol, heat and reflux at 75°C for 12h, cool to room temperature after the reaction is completed, spin the solvent to obtain a brown oily liquid, and use Acetone / ethyl acetate recrystallization gave 2-methyl-3-benzyloxypyridone as a light yellow solid. Yield 75%.
[0086] Add 2,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com