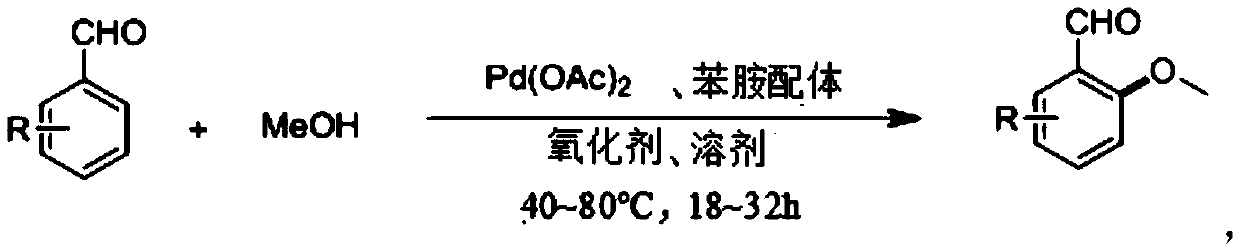
A kind of preparation method of o-methoxybenzaldehyde
A technology of o-methoxybenzaldehyde and methoxybenzaldehyde is applied in the field of preparation of o-methoxybenzaldehyde, and can solve the problems of complex synthesis method of o-methoxybenzaldehyde, low product yield and purity, and reaction conditions. Harsh and other problems, to achieve the effect of reducing reaction temperature and solvent consumption, high yield and purity, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
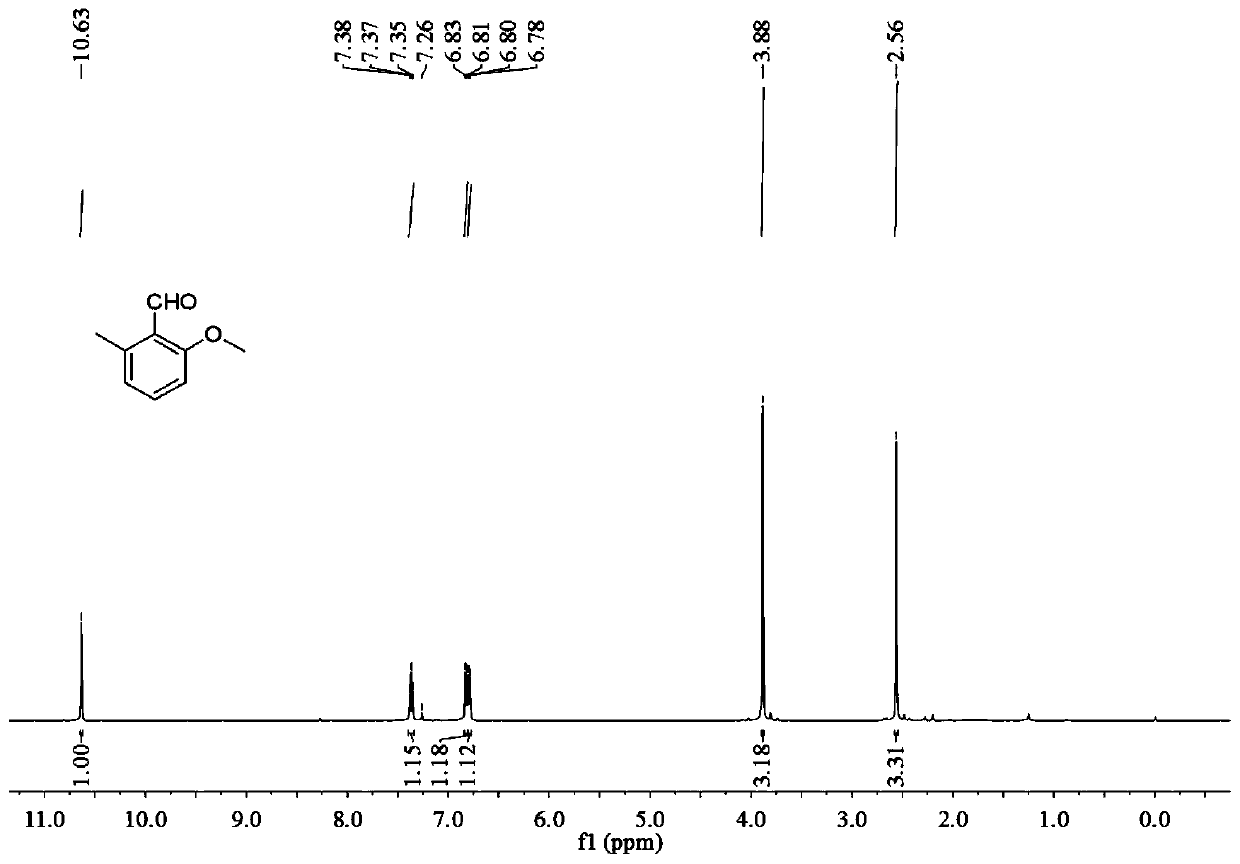
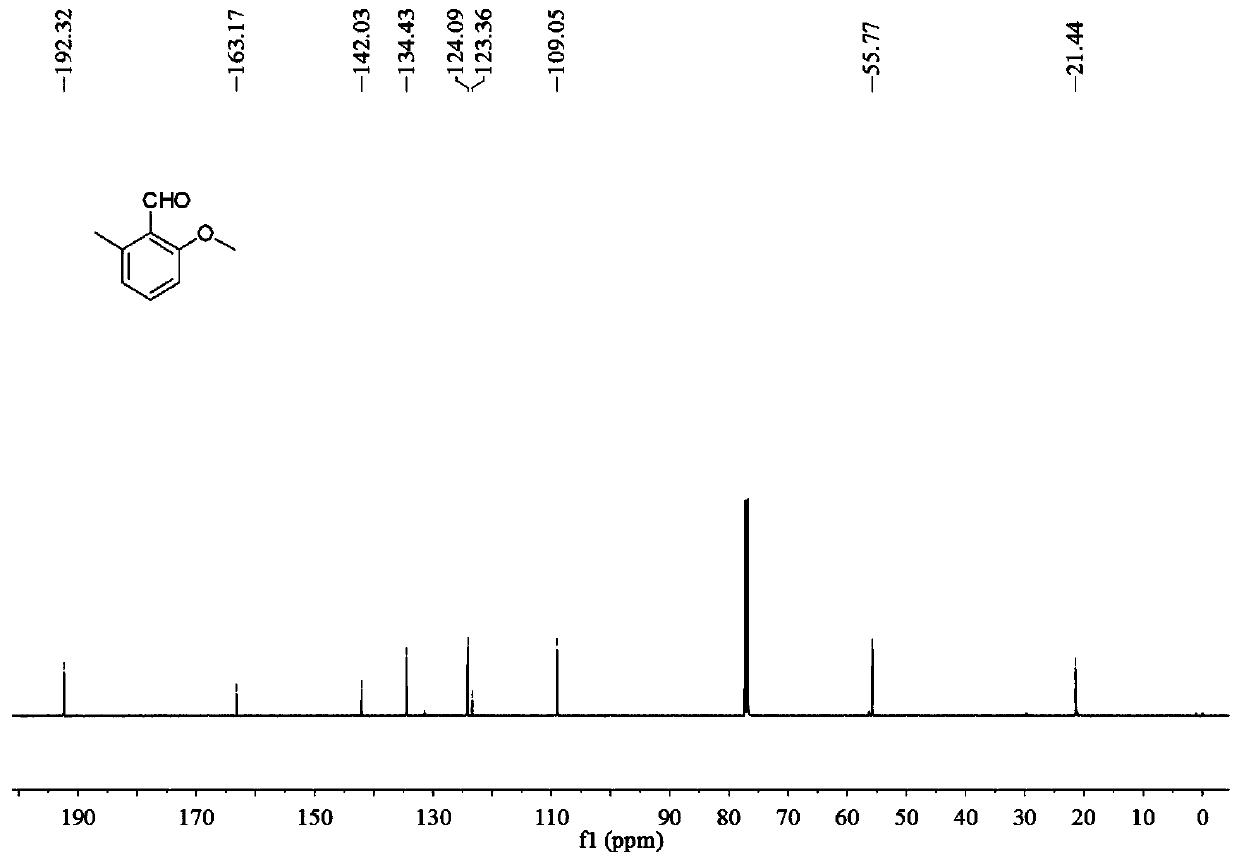
Image
Examples
Embodiment 1
[0037] A preparation method for o-methoxybenzaldehyde, specifically comprising the following steps:
[0038] 1) 12.0mg (0.1mmol) of o-tolualdehyde, 64.0mg (20.0mmol) of anhydrous methanol (MeOH), 2.25mg (0.01mmol) of palladium acetate (Pd(OAc) 2 ), 54.0mg (0.2mmol) potassium persulfate (K 2 S 2 o 8 ), 6.44mg (0.04mmol) of m-trifluoromethylaniline (TDG) were mixed in 1ml of dichloromethane (DCM), and then stirred and reacted on a magnetic stirrer at 60°C for 24h, that is, in o-tolualdehyde The methoxylation reaction of the ortho position of the aldehyde group obtains the reaction solution A. During the reaction, the specification can be -254 thin-layer chromatographic plate spot plate to track whether the reaction is complete;
[0039] 2) filter the reaction solution A, and add saturated sodium bicarbonate aqueous solution and dichloromethane to the filtered filtrate B for extraction to obtain the lower layer extract C, wherein the volume ratio of saturated sodium bicarbon...
Embodiment 2
[0056] The difference between this embodiment and Example 1 is that in this embodiment, the aniline ligand that condenses with the aldehyde group in o-tolualdehyde to form a transient directing group is o-trifluoromethylaniline, and the amount of o-trifluoromethylaniline Be 6.44mg (0.04mmol), other preparation raw material composition and the preparation process of o-methoxybenzaldehyde are the same as embodiment 1.
[0057] The amount of o-methoxybenzaldehyde obtained in this embodiment is 6.75 mg, and the calculated yield is 45%.
Embodiment 3
[0059] The difference between this embodiment and Example 1 is: in this embodiment, the oxidant is sodium persulfate, and its consumption is 47.6 mg (0.2 mmol), and the composition of other preparation raw materials and the preparation process of o-methoxybenzaldehyde are the same as in Example 1.
[0060] The amount of o-methoxybenzaldehyde obtained in this example was 7.35 mg, and the calculated yield was 49%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com