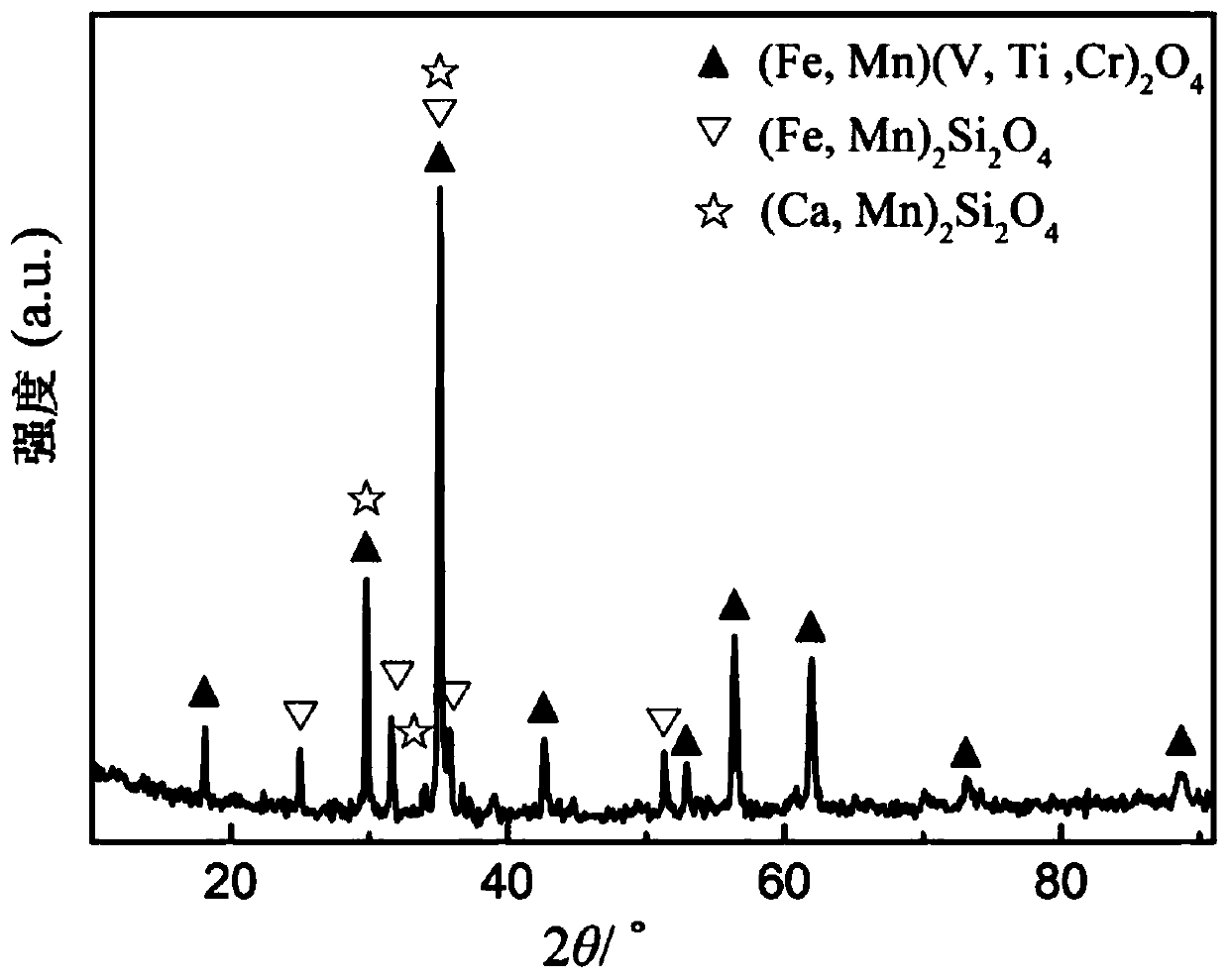
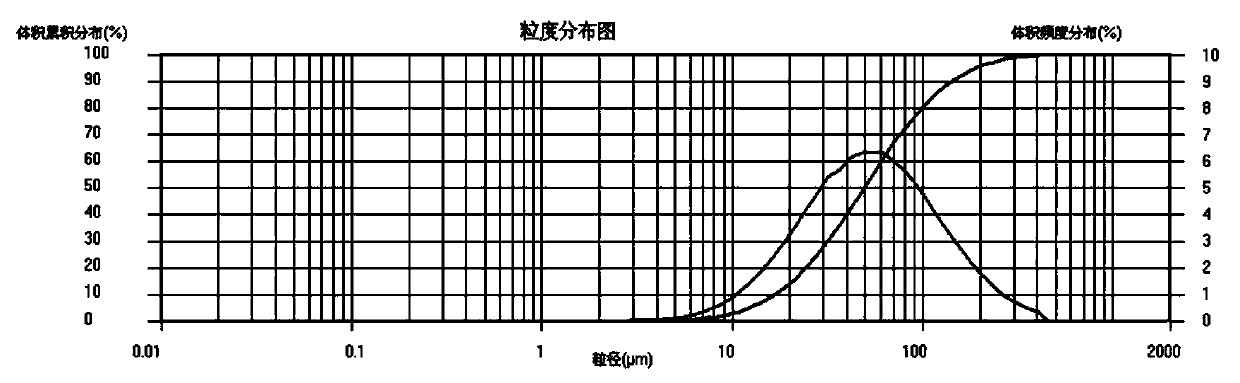
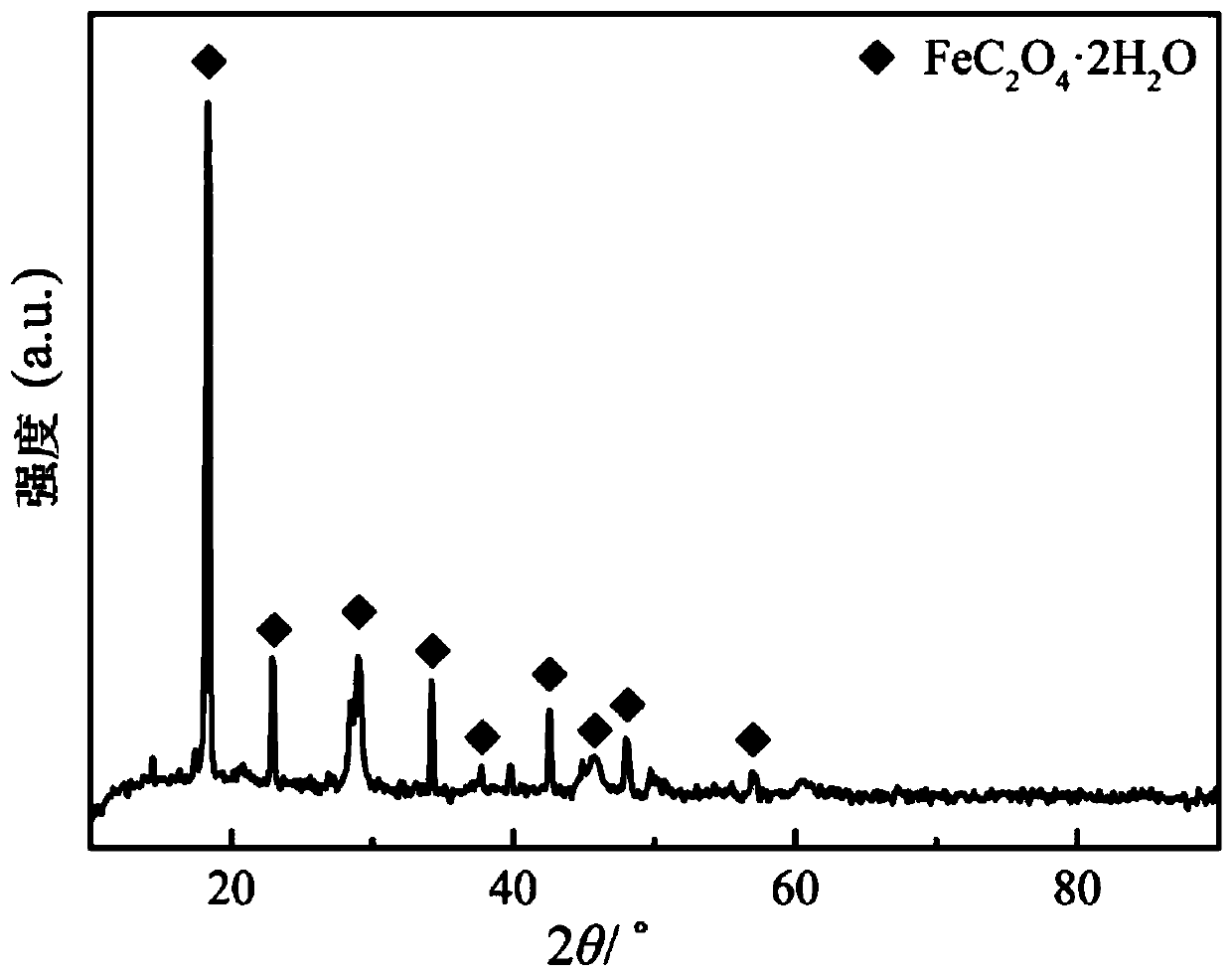
Method for preparing ferrous oxalate by using vanadium slag
A technology of ferrous oxalate and vanadium slag, which is applied in the direction of carboxylate preparation, carboxylate preparation, chemical instruments and methods, etc., to achieve the effect of simple synthesis, low equipment requirements, and comprehensive utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A kind of method utilizing vanadium slag to prepare ferrous oxalate, its concrete steps are as follows:
[0051] (1) First, the quaternary ammonium salt and oxalic acid dihydrate are placed in a beaker to mix uniformly according to a molar ratio of 1:1 to form a deep eutectic solvent as a leaching agent, wherein the quaternary ammonium salt is choline chloride;
[0052] (2) Then the vanadium slag is added in the deep eutectic solvent (liquid-solid ratio is 1:15), the stirring speed is 400 rpm, the temperature is 333K, and the leaching time is 8h;
[0053] (3) Separating the leached pulp from solid to liquid, adding 4 times the volume of deionized water to the leachate obtained by filtration, and standing for precipitation for 3 hours;
[0054] (4) Finally, the leaching solution after standing and precipitating is filtered, and the generated powder is ultrasonically washed with deionized water, washed with alcohol, and vacuum-dried to obtain the higher-purity ferrous oxa...
Embodiment 2
[0057] A kind of method utilizing vanadium slag to prepare ferrous oxalate, its concrete steps are as follows:
[0058] (1) First, quaternary ammonium salt and dihydrate oxalic acid are placed in a beaker and mixed uniformly according to a molar ratio of 1:2 to form a deep eutectic solvent as a leaching agent, wherein the quaternary ammonium salt is choline chloride;
[0059] (2) Then the vanadium slag is added in the deep eutectic solvent (liquid-solid ratio is 1:10), the stirring speed is 500 rpm, the temperature is 323K, and the leaching time is 10h;
[0060] (3) Separating the leached pulp from solid to liquid, adding 4 times the volume of deionized water to the leachate obtained by filtration, and standing for precipitation for 5 hours;
[0061] (4) Finally, the leaching solution after static precipitation is filtered, and the generated powder is ultrasonically washed with deionized water, washed with alcohol, and vacuum-dried to obtain the higher-purity ferrous oxalate c...
Embodiment 3
[0064] A kind of method utilizing vanadium slag to prepare ferrous oxalate, its concrete steps are as follows:
[0065] (1) First, the quaternary ammonium salt and oxalic acid dihydrate are placed in a beaker to mix uniformly according to a molar ratio of 1:1 to form a deep eutectic solvent as a leaching agent, wherein the quaternary ammonium salt is choline chloride;
[0066] (2) Then vanadium slag is added in the deep eutectic solvent (liquid-solid ratio is 1:10), stirring speed is 500 rpm, temperature is 353K, and leaching time is 4h;
[0067] (3) Separating the leached pulp from solid to liquid, adding 4 times the volume of deionized water to the leachate obtained by filtration, and standing for precipitation for 2 hours;
[0068] (4) Finally, the leaching solution after static precipitation is filtered, and the generated powder is ultrasonically washed with deionized water, washed with alcohol, and vacuum-dried to obtain the higher-purity ferrous oxalate crystal, which ha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com