Method using enzymolysis method to recycle kanamycin A from amikacin treating liquid
A kanamycin and amikacin technology, applied in the field of medicinal chemistry, can solve the problems of increased energy consumption for recovery, difficulty in operation, increased difficulty in production, and low recovery rate, and achieves easy industrial production, easy crystallization and separation, and reduced The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the preparation of amikacin synthetic liquid
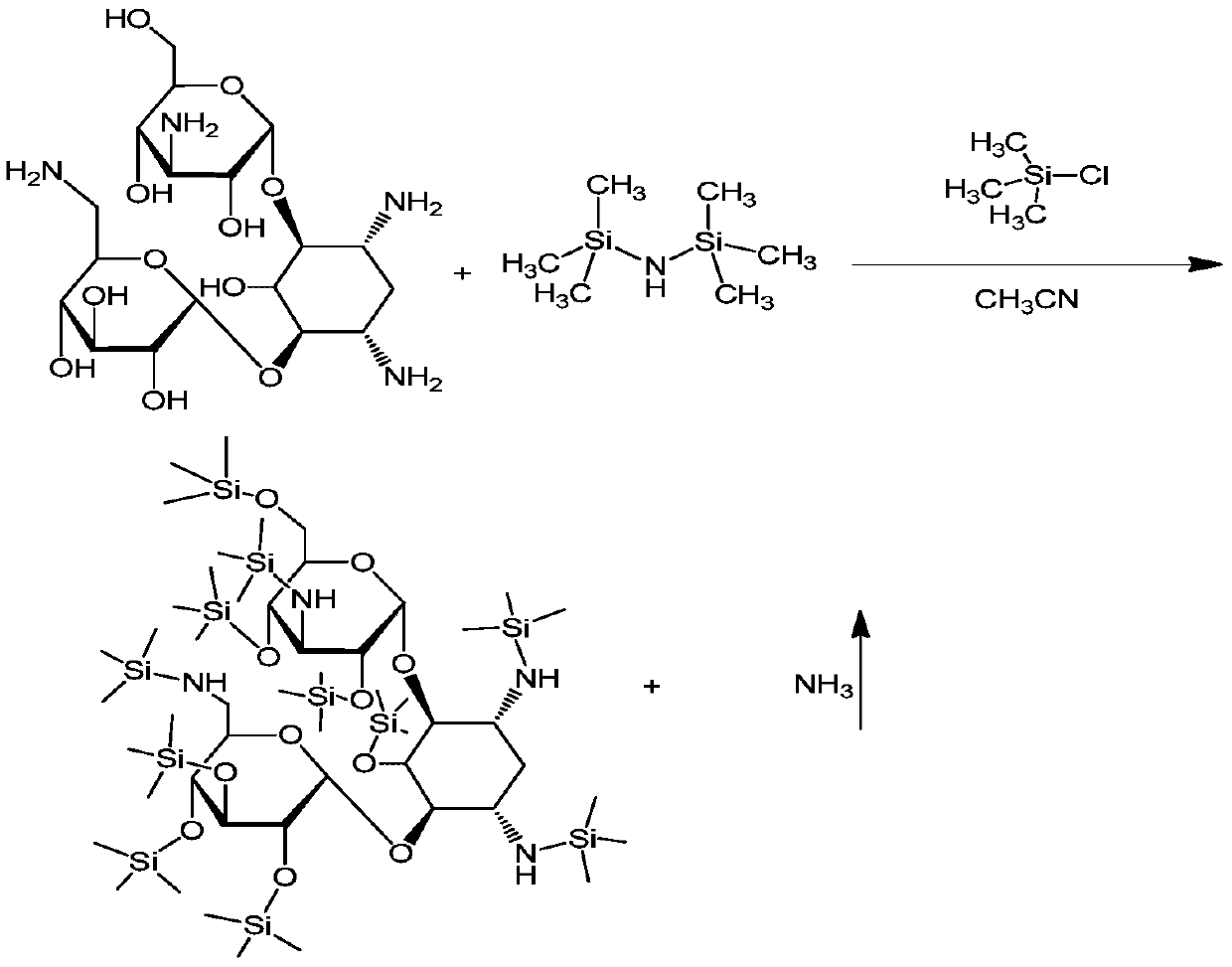
[0038] Put 600m of acetonitrile into the silanization reaction bottle, put in 132g of kanamycin A (KMA), close the feeding port and start stirring for 10 minutes, then add 400mL of hexamethyldisilazane (HMDS), heat up to reflux, at 75~ Reflux reaction at 80°C for 7hr. After the reaction was completed, the temperature was lowered to below 35°C, left to stand, and the layers were separated naturally. The lower layer was separated and collected to obtain a silyl-protected product.
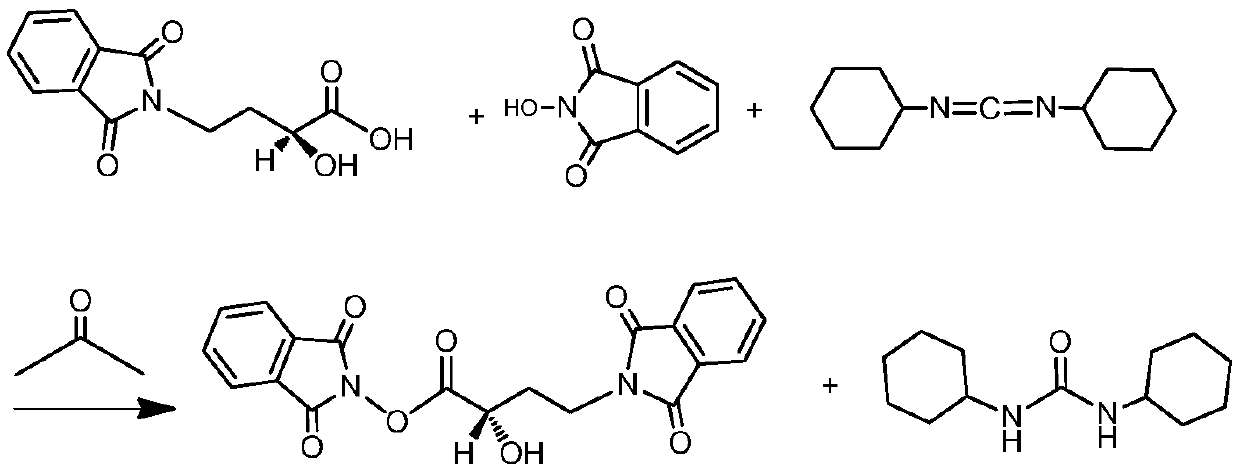
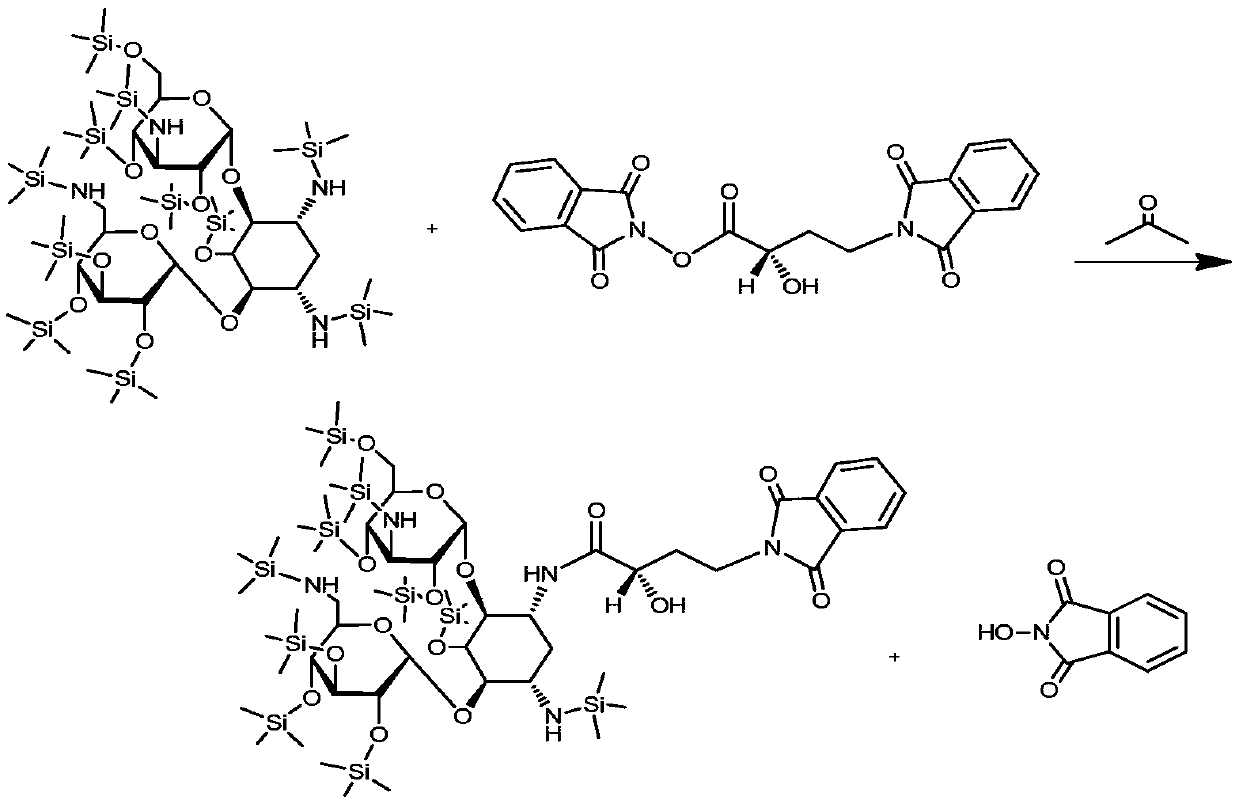
[0039] Add 1000mL of acetone to the silyl-protected product, start stirring, add 60g of γ-N-phthalimide-α-hydroxybutyric acid (PHBA), and then add 2.5g of catalyst 4-N,N-dimethyl Pyridine (DMAP), lower the temperature to -15°C to -10°C. Dissolve 60g of N,N-dicyclohexylcarbodiimide in 300mL of acetone, slowly add it to the above reactant at a controlled speed of 5mL / min, and control the temperature of the reactant at -15°C to -10°C;...
Embodiment 2
[0043] Embodiment 2: Purification treatment of amikacin synthetic liquid
[0044] 1510 mL of the amikacin synthetic solution obtained by the preparation method in Example 1 was purified by CD180 macroporous cation exchange resin, and eluted with ammonia water with a concentration of 0.5 mol / L, and the pure amikacin eluate and cardamom were collected respectively. The Namycin A eluate and the K29 eluate were concentrated to obtain 85.9 g of amikacin, 29.6 g of crude Kanamycin A, and 26.2 g of the K29 fraction, respectively.
Embodiment 3
[0045] Embodiment 3: the recovery preparation of kanamycin A
[0046] Add 26.2 g of the obtained K29 component into a 1 L reaction vessel, add 130 ml of purified water, and stir to dissolve. Control the pH value of the feed solution to 7.8; add 0.26 g of immobilized enzyme CALB, and then stir and react at room temperature for 3 hours to generate a reaction solution containing kanamycin A; after the reaction is completed, filter and recover the immobilized enzyme CALB. Use 5M sulfuric acid in the filtrate to adjust the pH value of the reaction solution to 4.5, add 520ml of ethanol, cool down to 0-10°C, stir and crystallize for 3 hours; after crystallization, add concentrated ammonia water and stir to adjust the pH value to neutral, then filter and collect the solid 20.1g, the crude product of kanamycin A.
[0047]Combined with the 29.6g Kanamycin A crude product obtained in Combining Example 2, a total of 49.7g, with a purity of 96.0%. The above crude product can also be re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com