Catalyst for electrochemical reduction of carbon dioxide and preparation method of catalyst
A carbon dioxide and catalyst technology, which is applied in the field of carbon dioxide electrochemical reduction catalyst and its preparation, can solve the problems of reducing the yield of formic acid, high overpotential, and accelerating the rate of hydrogen evolution, and achieve the goal of improving energy efficiency, high electrocatalytic activity and selectivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
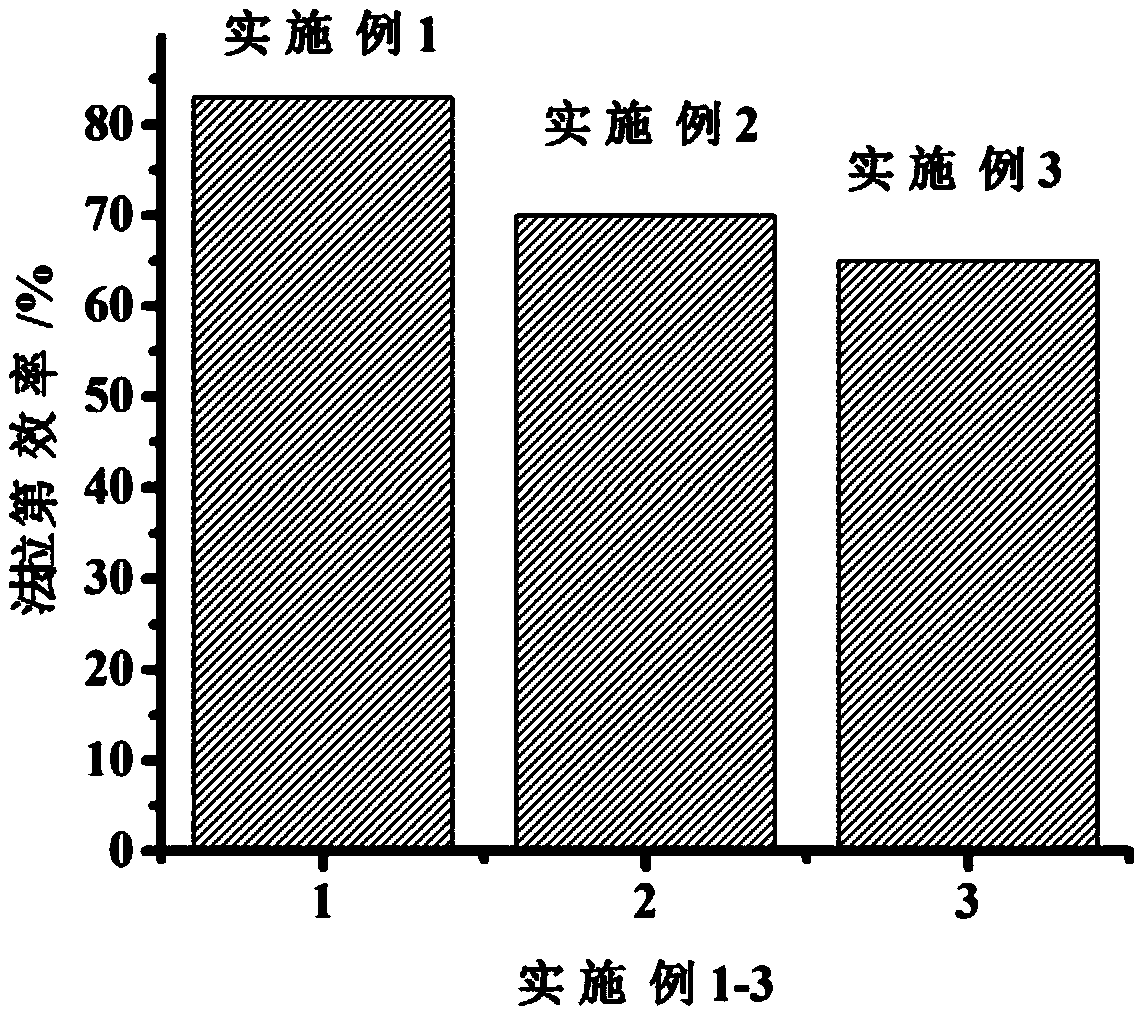
Embodiment 1
[0019] The preparation method of the carbon dioxide electrochemical reduction catalyst includes the following steps:
[0020] (1) Precursor preparation by spray drying method: Weigh 0.2 g of copper chloride and dissolve it in 150 mL of deionized water to obtain a copper chloride aqueous solution; under stirring conditions, add the copper chloride aqueous solution to 150 mL with a concentration of 2 mg / mL In the graphene oxide aqueous solution, stir evenly to obtain a mixed solution; the mixed solution is spray-dried at a temperature of 180°C and a feed rate of 5 mL / min, and the product is collected to obtain a precursor;
[0021] (2) Calcining to prepare composite materials: Take 0.3 g of the precursor prepared in step (1) and spread it on the bottom of the porcelain boat, place it in a tube furnace for calcination, and pass argon at a rate of 150 mL / min and keep argon throughout the process , The temperature is raised to 250 DEG C at a heating rate of 2 DEG C / min, the temperature ...
Embodiment 2
[0023] The preparation method of the carbon dioxide electrochemical reduction catalyst includes the following steps:
[0024] (1) Precursor preparation by spray drying method: Weigh 0.1g of copper chloride and dissolve it in 100mL of deionized water to obtain an aqueous solution of copper chloride; under stirring conditions, add the aqueous solution of copper chloride to 100mL with a concentration of 1mg / mL In the graphene oxide aqueous solution, stir evenly to obtain a mixed solution; spray-dry the mixed solution at a temperature of 150°C and a feed rate of 1 mL / min, and collect the product to obtain a precursor;
[0025] (2) Calcining to prepare composite materials: take 0.1g of the precursor prepared in step (1) and spread it on the bottom of the porcelain boat, place it in a tube furnace for calcination, and pass in argon at a rate of 100mL / min and keep argon throughout the process , The temperature is raised to 200°C at a temperature rise rate of 1°C / min, the temperature is ke...
Embodiment 3
[0027] The preparation method of the carbon dioxide electrochemical reduction catalyst includes the following steps:
[0028] (1) Precursor preparation by spray drying method: Weigh 0.5 g of copper chloride and dissolve it in 200 mL of deionized water to obtain a copper chloride aqueous solution; under stirring conditions, add the copper chloride aqueous solution to 200 mL with a concentration of 5 mg / mL In the graphene oxide aqueous solution, stir evenly to obtain a mixed solution; spray-dry the mixed solution at a temperature of 200°C and a feed rate of 10 mL / min, collect the product, and obtain a precursor;
[0029] (2) Calcining to prepare composite materials: Take 1g of the precursor prepared in step (1) and spread it on the bottom of the porcelain boat, place it in a tube furnace for calcination, and pass argon at a rate of 200 mL / min and keep the argon for the whole process. The temperature is raised to 300°C at a temperature rise rate of 5°C / min, and the temperature is kept...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com