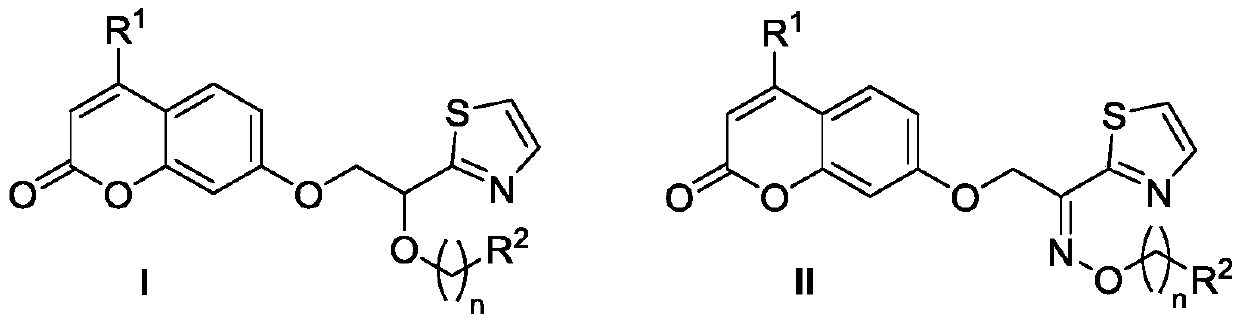
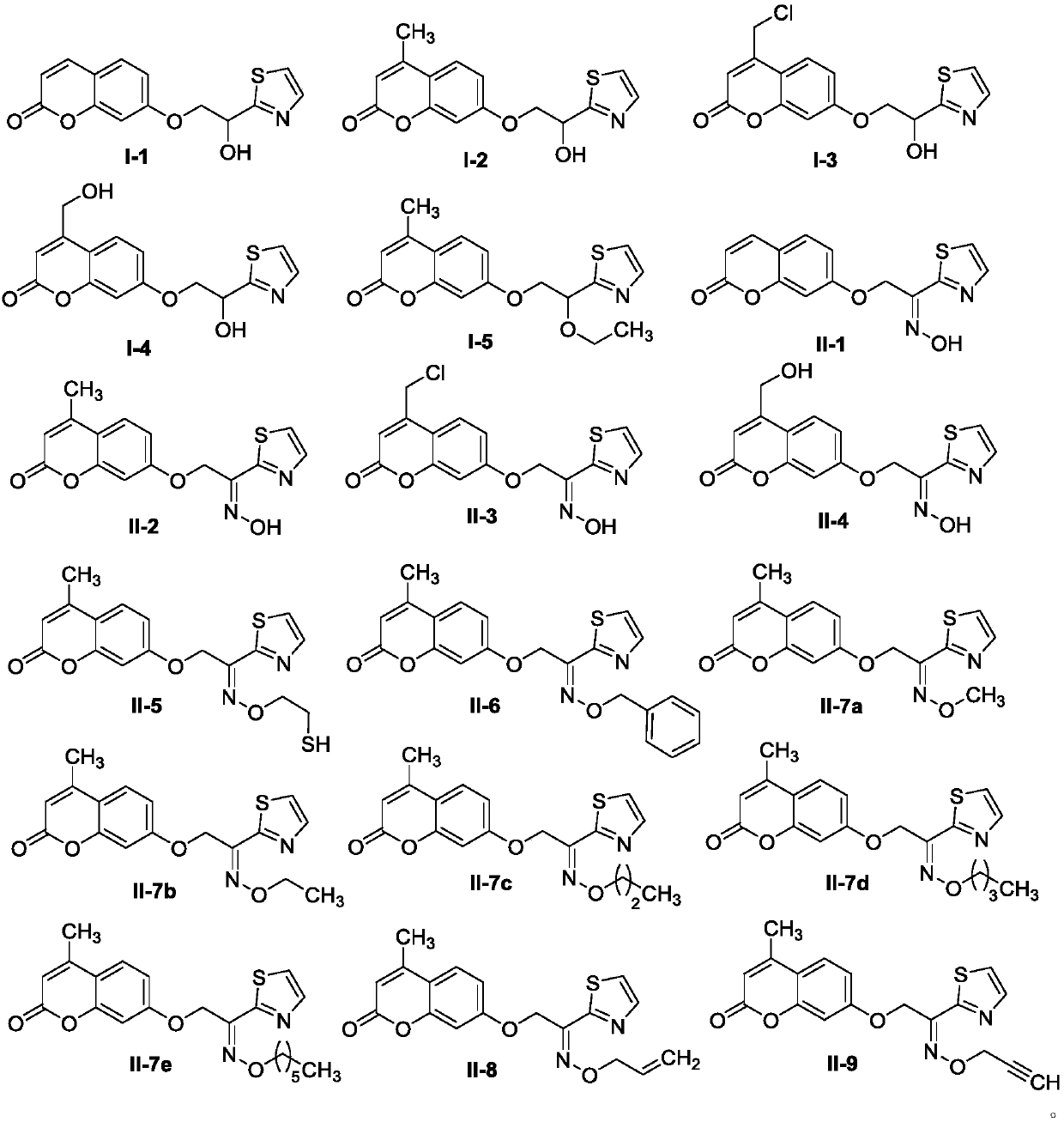
Ethoxy bridged thiazole coumarin compounds and preparation method and application thereof
A technology of ethoxy bridging and coumarin, applied in medical preparations containing active ingredients, organic chemistry, pharmaceutical formulations, etc., to achieve the effect of solving drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
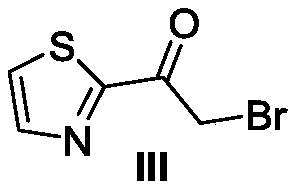
[0061] Preparation of Intermediate III
[0062]
[0063] Add 2-acetylthiazole (15.0g, 0.12mol) into a 100mL round bottom flask, use glacial acetic acid (30mL) as solvent, bromine (21.5g, 0.135mol) is diluted with glacial acetic acid in the dropping funnel and slowly drop into Stir the reaction at 50°C in a round-bottomed flask, absorb the tail gas from a saturated sodium carbonate solution, and follow up with thin-layer chromatography until the end of the reaction. After cooling to room temperature, a light yellow solid was precipitated, filtered with suction, and the filter residue was washed twice with ether, dried and other post-treatments to obtain Intermediate III (18.0 g), with a yield of 73.2%.
experiment example 2
[0065] Preparation of Intermediate IV
[0066]
[0067] Add 7-hydroxycoumarin (720mg, 4.4mmol), potassium carbonate (670mg, 4.88mmol) and intermediate III (1.0g, 4.9mmol) into a 50mL round bottom flask, with acetonitrile (25mL) as solvent, 60°C The reaction was stirred under low pressure, followed by TLC until the end of the reaction, cooled to room temperature, separated by column chromatography, dried and other post-treatments to obtain intermediate IV (700 mg), yield 54.9%; white solid; melting point: 179-180 ° C, Among them, 7-hydroxycoumarin is obtained by Pechmann reaction with resorcinol as the starting material. 1 H NMR (600MHz, DMSO-d 6 )δ8.33(d, J=2.2Hz, 1H, thiazolyl-4-H), 8.24(d, J=2.4Hz, 1H, thiazolyl-5-H), 8.00(d, J=9.4Hz, 1H, coumarin-4-H), 7.65 (d, J = 8.6Hz, 1H, coumarin-5-H), 7.09 (s, 1H, coumarin-6-H), 7.04 (dd, J = 8.6, 1.9Hz, 1H , coumarin-8-H), 6.31 (d, J=9.5Hz, 1H, coumarin-3-H), 5.80 (s, 2H, OCH 2 )ppm.
experiment example 3
[0069] Preparation of Intermediate V
[0070]
[0071] 4-Methyl-7-hydroxycoumarin (880mg, 5.0mmol), potassium carbonate (691mg, 5.5mmol) and Intermediate III (1.1g, 5.5mmol) were added to a 50mL round bottom flask and dissolved in acetonitrile (25mL) As a solvent, stir the reaction at 60°C, follow up to the end of the reaction by thin-layer chromatography, cool to room temperature, and obtain intermediate V (800 mg) after separation by column chromatography and drying, with a yield of 53.2%; pink solid; melting point: 225-226 ° C, wherein 4-methyl-7-hydroxycoumarin is obtained by Pechmann reaction with resorcinol as the starting material. 1 H NMR (600MHz, DMSO-d 6 )δ8.32(d, J=3.0Hz, 1H, thiazolyl-4-H), 8.23(d, J=2.9Hz, 1H, thiazolyl-5-H), 7.69(d, J=8.6Hz, 1H, coumarin-5-H), 7.09–7.03 (m, 2H, coumarin-6, 8-H), 6.22 (s, 1H, coumarin-3-H), 5.80 (s, 2H, OCH 2 ),2.40(s,3H,CH 3 )ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com