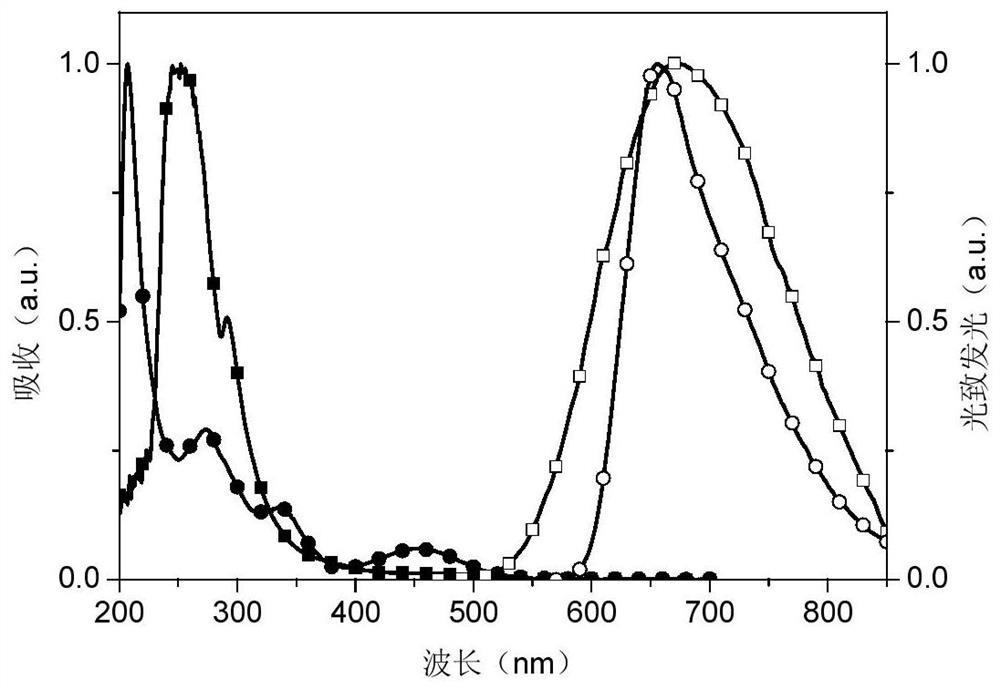
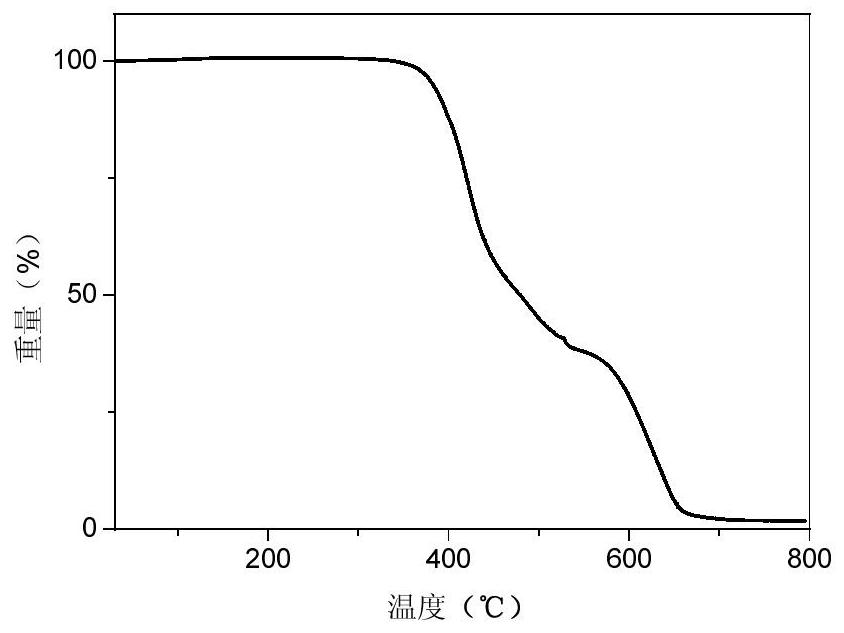
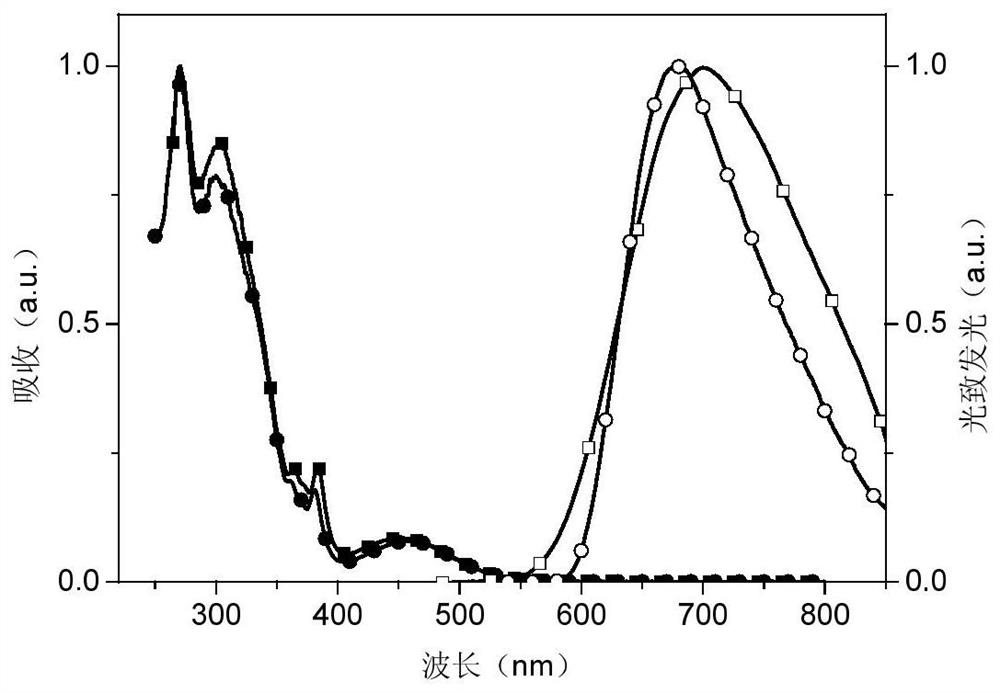
Phosphinoxy group-modified dipyridophenazinyl red light/near-infrared thermally excited delayed fluorescence material, synthesis method and application thereof
A near-infrared heat and delayed fluorescence technology, which is applied in the direction of luminescent materials, chemical instruments and methods, and compounds of group 5/15 elements of the periodic table, can solve the problems of low efficiency of light-emitting devices, concentration quenching, and fast decay. Reach the effect of reducing quenching effect, improving efficiency and inhibiting intermolecular interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0067] Specific Embodiment 1: The structural formula of the dipyridoxphenazinyl red light / near-infrared thermally excited delayed fluorescent material modified with phosphine groups in this embodiment is as follows: where X is hydrogen or Y is hydrogen,
[0068] Z is hydrogen, W is hydrogen or M is hydrogen or V is hydrogen or
[0069] N is hydrogen or U is hydrogen or
[0070] When W, Y, Z, M, V are hydrogen, X is N and U are , its structural formula is:
[0071] When W, Z, M, V are hydrogen, X, Y are N and U are , its structural formula is:
[0072] When X, Y, Z, M, V are hydrogen, W is N and U are , its structural formula is:
[0073] When X, Y, M, V are hydrogen, W, Z are N and U are , its structural formula is:
[0074] When W, Z, M, V are hydrogen, X is Y, N, U are , its structural formula is:
[0075] When X, Y, M, V are hydrogen, W is Z, N, U are , its structural formula is:
[0076] When W, Y, Z, N, U are hydr...
specific Embodiment approach 2
[0082] Specific embodiment 2: The synthesis method of the dipyridoxphenazinyl red light / near-infrared thermal excitation delayed fluorescent material modified by the phosphine group described in the specific embodiment 1 is carried out according to the following steps:
[0083] 1. Take 0.8~1.2mmol of 2,9-dibromophenanthroline-5,6-dione or 3,8-dibromophenanthroline-5,6-dione, 1mmol of the reaction precursor and ethanol 5 Mix ~10ml, react at 80°C for 48h, extract with water and dichloromethane, combine the organic layers, dry, and purify by column chromatography using a mixed solvent of dichloromethane and ethyl acetate as eluent to obtain the intermediate;
[0084] 2. Take 1 mmol of the intermediate synthesized in step 1, 0.05 mmol of palladium acetate, 1 to 10 mmol of sodium acetate, 1 mmol of diphenylphosphine and 5 to 10 ml of N,N dimethylformamide, mix them at 150°C for 48 hours, and add 5 mmol h 2 o 2 Oxidation, extraction with water and dichloromethane, combined organic...
specific Embodiment approach 3
[0085] Specific embodiment 3: The difference between this embodiment and specific embodiment 2 is that the reaction precursor described in step 1 is N4', N4'-diphenyl-[1,1'-biphenyl]-3,4,4 'Triamine, N4', N4'-diphenyl-[1,1'-biphenyl]-2,3,4'Triamine, N4, N4, N4', N4"-tetraphenyl[1,1 ':2',1"-terphenyl]-4,4',4",5'-tetramine, N4,N4,N4',N4"-tetraphenyl[1,1':4',1" -terphenyl]-2',3',4,4"-tetramine, (4,5-diamino-4'-(diphenylamino)-[1,1'-biphenyl]-2- Base) diphenylphosphine oxide, (2,3-diamino-4'-(diphenylamino)-[1,1'-biphenyl]-4-yl)diphenylphosphine oxide, N4', N4'-diphenyl-[1,1'-biphenyl]-3,4,4'triamine, N4',N4'-diphenyl-[1,1'-biphenyl]-2,3, 4'triamine, N4, N4, N4', N4"-tetraphenyl[1,1':2',1"-terphenyl]-4,4', 4", 5'-tetramine, N4, N4, N4', N4"-tetraphenyl[1,1':4',1"-terphenyl]-2',3',4,4"-tetramine, (4,5-diamino-4 '-(diphenylamino)-[1,1'-biphenyl]-2-yl)diphenylphosphine oxide or (2,3-diamino-4'-(diphenylamino)-[1 ,1'-biphenyl]-4-yl)diphenylphosphine oxide. Others are the same as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com