Method of preparing hyperbranched polythioether
A technology of polysulfide and trithiol, which is applied in the field of preparing hyperbranched polysulfide, can solve the problem of simultaneously regulating and controlling hyperbranched polysulfide skeleton and end groups, limiting the function and application field of hyperbranched polysulfide, Limit the preparation and application of hyperbranched polysulfides, and achieve the effects of fast polymerization rate, high controllability and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0027] Add 9.965g (25mmol) trimethylolpropane-tris(3-mercaptopropionate), 75mL tetrahydrofuran (THF) and 3.56g (25mmol) glycidyl methacrylate and 0.1012g (1.0mmol) triethylamine once added into the reactor, at room temperature N 2 Protect the reaction for 24 hours; after the reaction, the reaction solution was concentrated by rotary evaporation, redissolved in chloroform and precipitated in anhydrous ether, and the dissolution-precipitation operation was repeated 3 times, and the precipitate was vacuum-dried to obtain a colorless viscous mercapto-terminated hyperbranched polysulfide P1 (9.50 g, 70.4% yield).
Embodiment example 2
[0029] 9.965g (25mmol) trimethylolpropane-tris(3-mercaptopropionate), 75mL N,N-dimethylformamide (DMF), 3.56g (25mmol) glycidyl methacrylate and 0.1222g (1.0mmol) N, N-lutidine was added to the reactor at one time, and N 2 Protect the reaction for 24 hours; after the reaction, the reaction solution was concentrated by rotary evaporation, redissolved in chloroform and precipitated in anhydrous ether, and the dissolution-precipitation operation was repeated 3 times, and the precipitate was vacuum-dried to obtain a colorless viscous mercapto-terminated hyperbranched polysulfide P2 (9.26 g, 68.5% yield).
Embodiment example 3
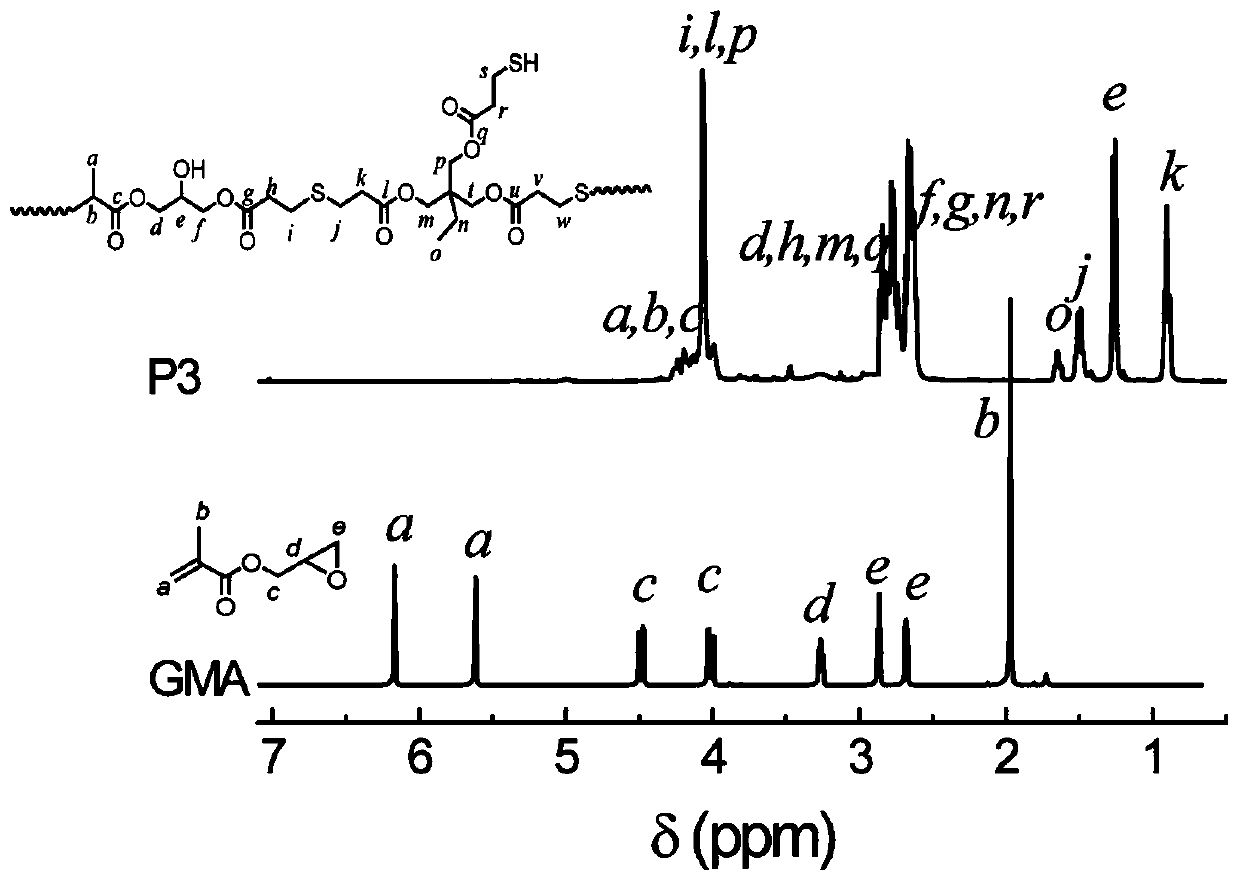
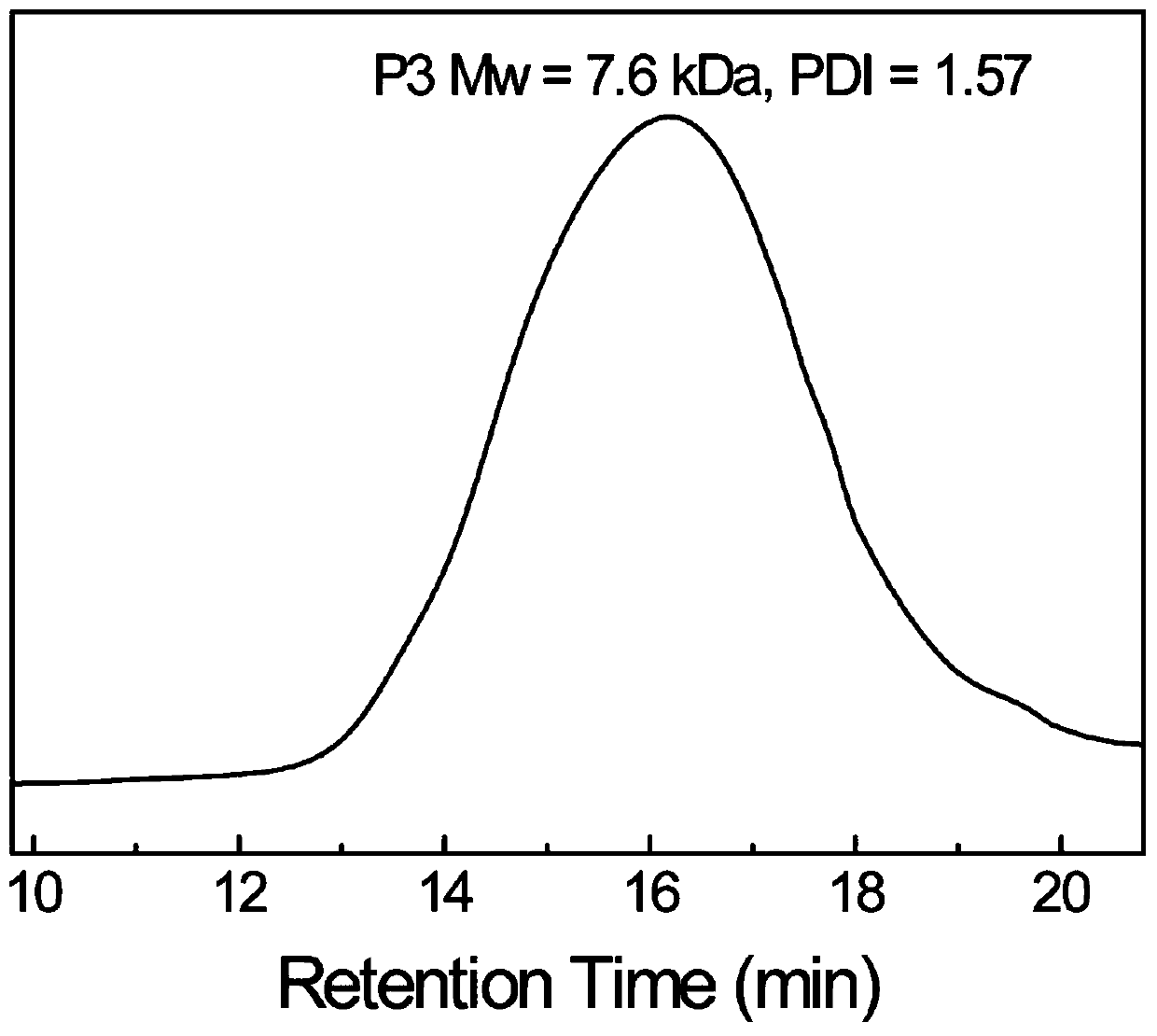
[0031] 9.965g (25mmol) of trimethylolpropane-tris(3-mercaptopropionate), 75mL of DMF and 3.56g (25mmol) of glycidyl methacrylate and 0.1522g (1.0mmol) of 1,8-diaza Bicyclo[5,4,0]undec-7-ene (DBU) was added to the reactor at one time, and N 2 Protect the reaction for 24 hours; after the reaction, the reaction solution was concentrated by rotary evaporation, redissolved in chloroform and precipitated in anhydrous ether, and the dissolution-precipitation operation was repeated 3 times, and the precipitate was vacuum-dried to obtain a colorless viscous mercapto-terminated hyperbranched polysulfide P3 (8.57 g, 63.4% yield).
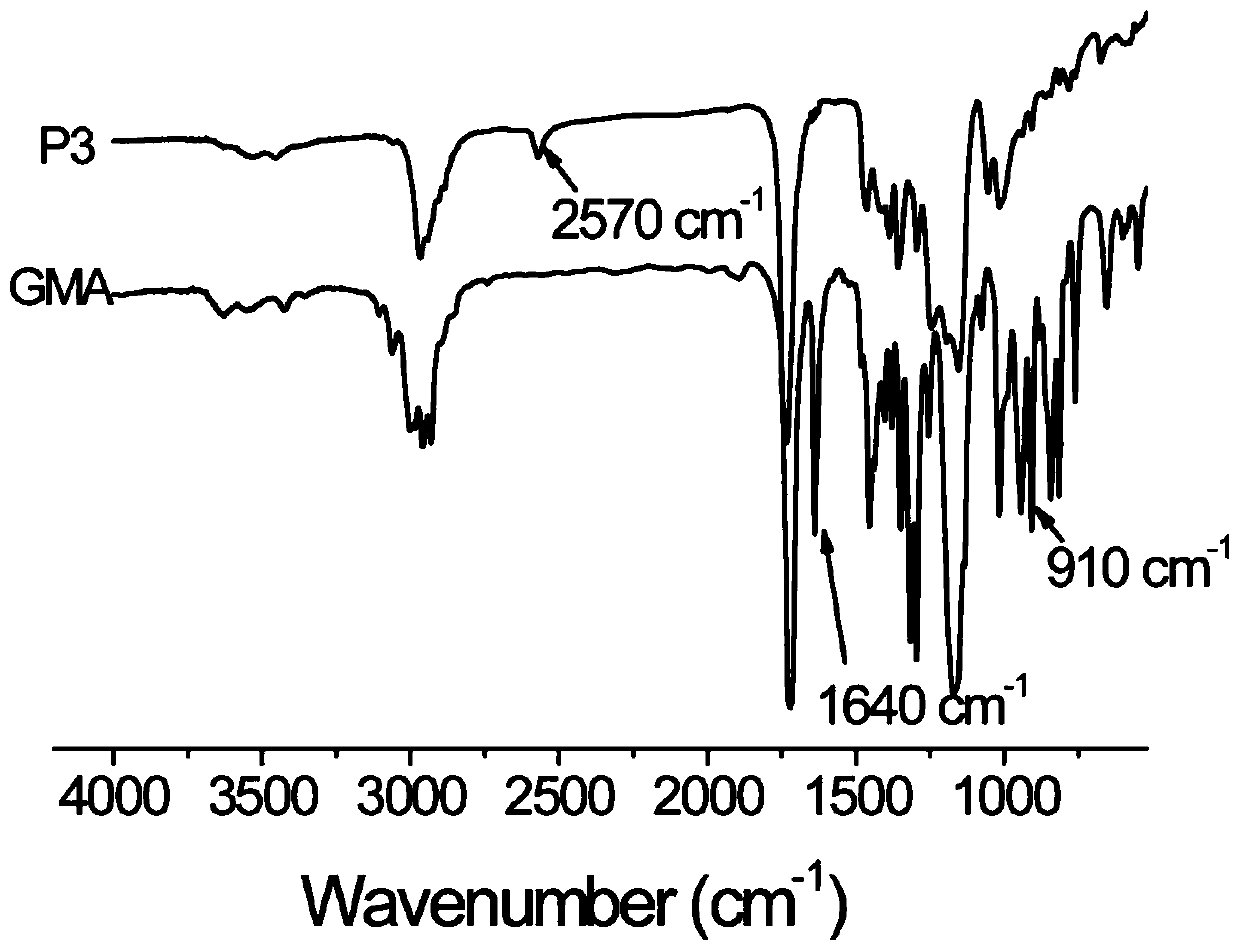
[0032] figure 1 It is the infrared absorption spectrogram of the mercapto-terminated hyperbranched polysulfide P3 and monomer glycidyl methacrylate (GMA) prepared by embodiment case 3, wherein 3505cm in the P3 spectrogram -1 The stretching vibration absorption peak of OH is at 2570cm -1 The characteristic absorption peak of thiol is at 910cm -1 The charact...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com