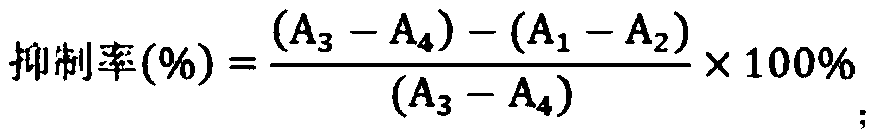Application of astaxanthin to preparation of aldose reductase inhibitor
A reductase inhibitor, astaxanthin technology, applied in the field of application of astaxanthin in the preparation of aldose reductase inhibitors, to achieve the effect of reducing the occurrence of diabetic complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 astaxanthin
[0029] The Phaffia fermented liquid was 5534×g, centrifuged for 8 min, the supernatant was discarded, washed with distilled water and centrifuged again, and the operation was repeated twice to obtain Phaffia cells.
[0030] Phaffia yeast cell wall breaking: Use dimethyl sulfoxide to break the Phaffia yeast cell wall, the breaking condition is: the cell and dimethyl sulfoxide are based on the mass-volume ratio, that is, g / ml, and the ratio is 1 / 2. At 55°C, break the wall for 5 minutes; using dimethyl sulfoxide has a high breaking rate and good extraction effect; the short treatment time (5min) and low treatment temperature (55°C) are conducive to the stability of the astaxanthin structure .
[0031] Crude extraction of astaxanthin with acetone: after breaking the wall, extract with acetone according to the ratio of material to liquid 1:15, the ratio of material to liquid is the mass-volume ratio of Phaffia thallus to aceton...
Embodiment 2
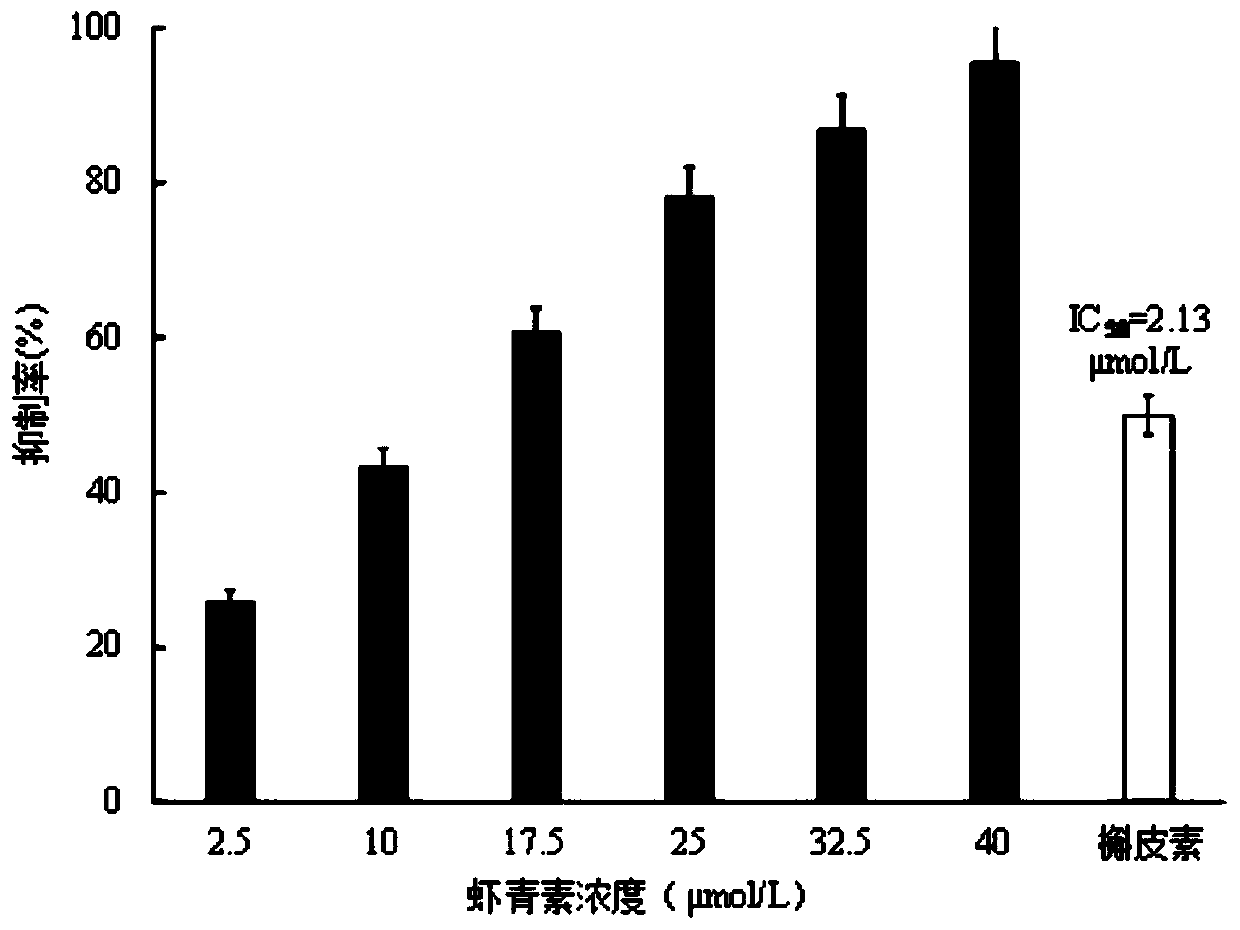
[0036] The measurement of the inhibitory rate of embodiment 2 astaxanthin to aldose reductase activity
[0037] Preparation of reagents:
[0038] Preparation of phosphate buffered saline (PBS): accurately weigh 6.36g NaH 2 PO 4 , add distilled water to dissolve, transfer to a 250mL volumetric flask to obtain liquid a; take another 3.31g Na 2 HPO 4 , add distilled water to dissolve, transfer to a 250mL volumetric flask to constant volume to obtain liquid b; then, pipette the two solutions of a and b to a 500mL volumetric flask to constant volume, use a pH meter to calibrate the phosphate buffer solution to pH=6.2 .
[0039] Preparation of aldose reductase solution: Take 100 U of aldose reductase powder and add 1 mL of PBS, that is, the enzyme activity of aldose reductase is 100 U / mL. Take 20 μL of 100 U / mL aldose reductase solution and add 980 μL of PBS, that is, the concentration of aldose reductase is 2 U / mL, and the aldose reductase solution should be prepared and used im...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com