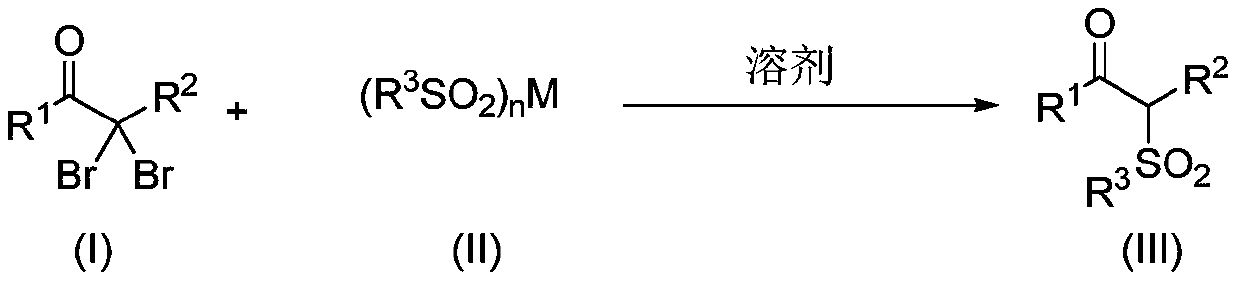Method for synthesizing alpha-sulfone ketone compound from alpha, alpha-dibromo ketone by one-pot process
A ketone compound and synthesis method technology, applied in the field of organic chemical synthesis, can solve the problems of low overall utilization rate, expensive reagents, cumbersome operation, etc., and achieve good industrialization prospects, potential application value, and the effect of increasing economic benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038]A magnetic stir bar, 2,2-dibromo-1-(4-methylphenyl)-1-propanone (61.2mg, 0.2mmol) and sodium p-toluenesulfinate (0.6mmol) were added to a Schlenk reaction tube , 3equiv.), and then added 1mL of anhydrous methanol under nitrogen protection, and stirred at 50°C for 12h. After the reaction was finished, the solvent was evaporated on a rotary evaporator, and the residue was purified by flash column chromatography (petroleum ether / ethyl acetate volume ratio was 5:1) to obtain the target product, with an isolation yield of 93%, and the product was a white solid powder , melting point m.p.104-105°C.
[0039] NMR: 1 H NMR (500MHz, CDCl 3 )δ7.88(d, J=7.8Hz, 2H), 7.65(d, J=7.8Hz, 2H), 7.37–7.21(m, 4H), 5.13(q, J=6.6Hz, 1H), 2.48– 2.33(m,6H),1.54(d,J=6.7Hz,3H); 13 CNMR (125MHz; CDCl 3 ) δ 192.1, 145.3, 145.2, 133.9, 133.1, 129.8, 129.5, 129.4, 129.4, 64.9, 21.7, 21.7, 13.2.
Embodiment 2
[0041]
[0042] A magnetic stirrer, 2,2-dibromo-1-phenyl-1-propanone (58.4mg, 0.2mmol) and sodium p-toluenesulfinate (0.6mmol, 3equiv.) were added to a Schlenk reaction tube, and then Add 1 mL of anhydrous methanol under nitrogen protection, and stir at 50° C. for 12 h. After the reaction was finished, the solvent was evaporated on a rotary evaporator, and the residue was purified by flash column chromatography (petroleum ether / ethyl acetate volume ratio was 5:1) to obtain the target product, with an isolation yield of 98%, and the product was a white solid powder , melting point m.p.93-95°C.
[0043] NMR: 1 H NMR (500MHz, CDCl 3 )δ7.98(d, J=7.3Hz, 2H), 7.66(d, J=8.2Hz, 2H), 7.61(t, J=7.4Hz, 1H), 7.48(t, J=7.8Hz, 2H) ,7.31(d,J=8.0Hz,2H),5.15(q,J=6.9Hz,1H),2.43(s,3H),1.56(d,J=6.9Hz,3H); 13 C NMR (125MHz; CDCl 3 ) δ 192.6, 145.3, 136.3, 134.0, 133.1, 129.8, 129.5, 129.2, 128.7, 65.1, 21.7, 13.2.
Embodiment 3
[0045]
[0046] A magnetic stirrer, 2,2-dibromo-1-(4-chlorophenyl)-1-propanone (65.3mg, 0.2mmol) and sodium p-toluenesulfinate (0.6mmol, 3equiv.), then added 1mL of anhydrous methanol under nitrogen protection, and stirred at 50°C for 12h. After the reaction was finished, the solvent was evaporated on a rotary evaporator, and the residue was purified by flash column chromatography (petroleum ether / ethyl acetate volume ratio was 5:1) to obtain the target product, with an isolation yield of 96%, and the product was a white solid powder , melting point m.p.126-127°C.
[0047] NMR: 1 H NMR (500MHz, CDCl 3 )δ7.93(d, J=8.4Hz, 2H), 7.63(d, J=8.0Hz, 2H), 7.44(d, J=8.4Hz, 2H), 7.31(d, J=8.0Hz, 2H) ,5.10(q,J=6.9Hz,1H),2.43(s,3H),1.54(d,J=6.9Hz,3H); 13 C NMR (125MHz; CDCl 3 )δ191.4, 145.5, 140.7, 134.7, 132.9, 130.6, 129.8, 129.6, 129.1, 65.2, 21.7, 13.1;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com