Novel streptomyces trypsin GM2938 and heterologous expression thereof
A technology of GM2938 and trypsin, applied in the direction of enzymes, peptidases, hydrolytic enzymes, etc., can solve the problems of low enzyme yield, single component, and difficulty in heterologous expression, and achieve good application prospects and low collagenase activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment one: the acquisition of trypsin GM2938 gene
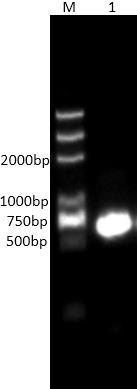
[0014] Genomic DNA of Streptomyces endophytic strain A249 was extracted, and the whole genome sequence was sequenced (completed by Beijing Novogene Biotech), combined with the obtained ORF reading frame, after reviewing relevant literature, comparing databases, and analyzing feasibility studies , a relatively novel trypsin GM2938 gene was selected, and its encoded amino acid sequence was 80.85% similar to the known trypsin SGT from Streptomyces griseus.
Embodiment 2
[0015] Example 2: Heterologous expression of GM2938 mature peptide gene
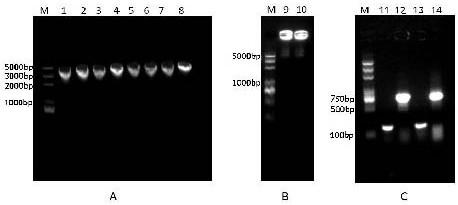
[0016] Design specific primers to amplify the mature peptide gene of GM2938, upstream primer (5'-AGGAGGCGCAACTCAAGCTTTTGCAGTCGTCGGCGGCACGCGCGCCG-3'), downstream primer (5'-AGCTTGCATGCCTGCAGGTCGACTCTCAGAGACCGGCGG CCGCTCTGGCT-3'), design plasmid pWB980 linearization primer, upstream primer (5'- AGCCAGAGCGGCCGCCGGTCTCTGAGAGTCGACCTGCAGGCATGCAAGCT-3'), downstream primers (5'-CGGCGCGCGTGCCGCCGACGACTGCAAAAGCTTGAGT TGCGCCTCCT-3'), obtained the amplified mature peptide gene of GM2938 and the linearized plasmid pWB980 fragment by PCR respectively, purified the recovered linearized plasmid and the target The gene fragments serve as primers for each other, and POE-PCR is carried out by ultra-high-fidelity enzymes, and the obtained plasmid polymer is transformed into the Bacillus subtilis engineering bacterium SCK6 to obtain the genetic engineering expression bacterium.
Embodiment 3
[0017] Embodiment three: Recombinant Bacillus subtilis engineered bacterium culture condition
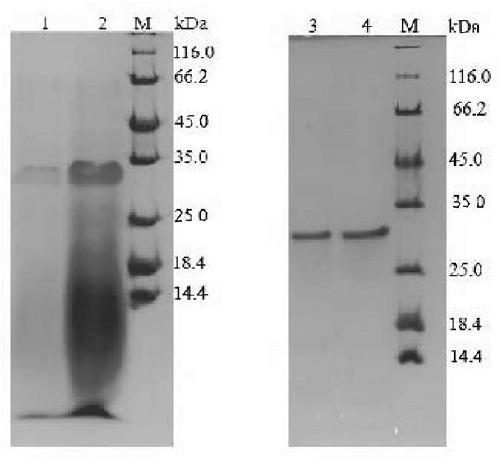
[0018] Engineering bacteria containing recombinant plasmid pWB980-mt2938 B. subtilis SCK6 was inoculated in 100 mL of LB-resistant medium containing 1 µg / mL erythromycin and 50 µg / mL kanamycin, cultured on a shaker at 37°C overnight, and the next day, the above seed solution was inoculated at a 2% inoculum In the fermentation medium with the same resistance (medium composition: 15g / L soluble starch, 16 g / L tryptone, 32 g / L soybean peptone, 72 mM K 2 HPO 4 , 17 mM KH 2 PO 4 ; the initial pH value of the medium was 8.0), 34 °C, 200rpm shaker culture for 72 h; since the promoters carried by the plasmid pWB980 are all constitutive promoters, no additional inducer is needed to induce protein expression, and the target gene is pre-expressed A signal peptide is added to promote the extracellular expression of the protein, so it is necessary to collect the supernatant obtained after c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com