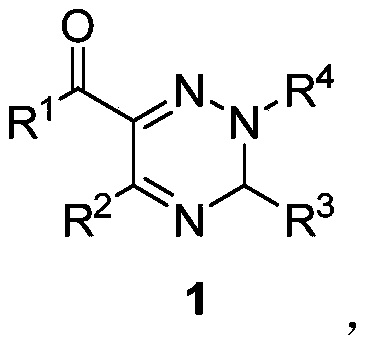
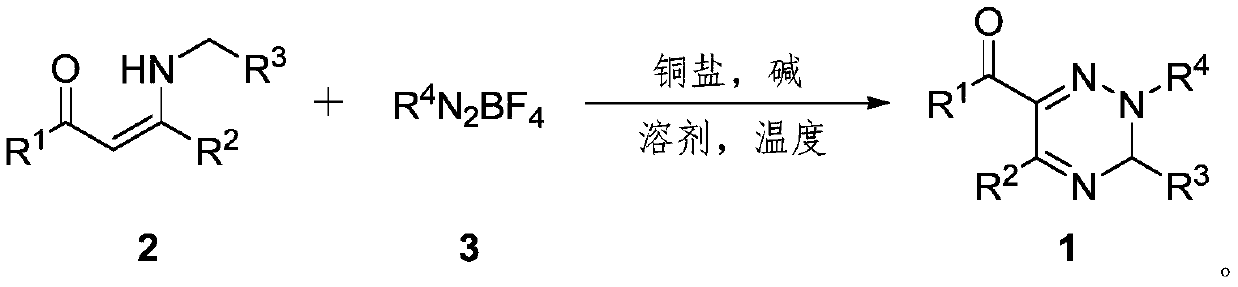
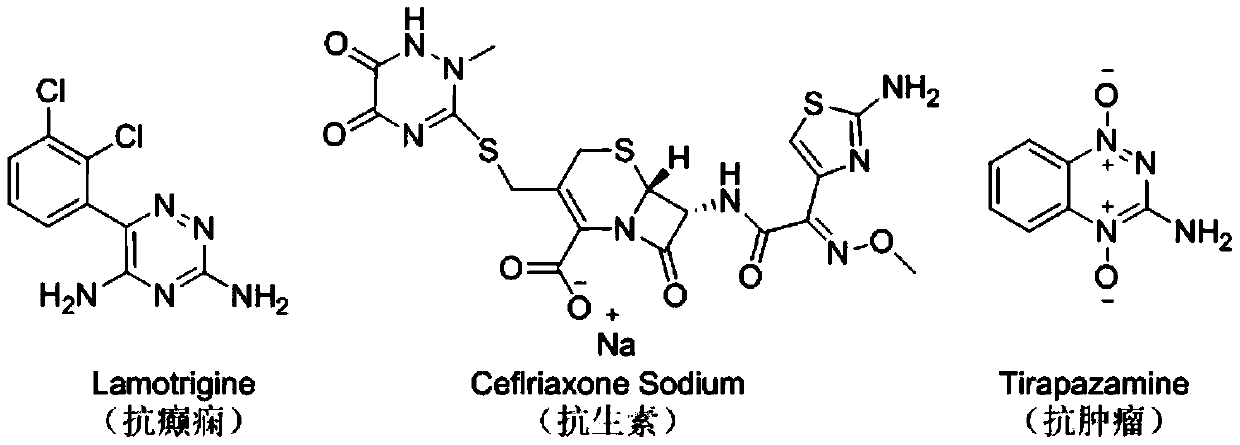
2,3,5,6-tetrasubstituted-3H-1,2,4-triazine derivative and synthesis method thereof
A technology for triazine derivatives and synthesis methods, applied in the direction of organic chemistry and the like, can solve the problems of harsh reaction conditions, limited types of substrates, poor regioselectivity, etc., and achieve the effects of low cost, cost advantage, and good functional group diversity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] Weigh successively α-carbonyl enamine 2a (0.5mmol), boron tetrafluoride benzene diazonium salt 3a (0.6mmol), copper bromide (0.05mmol), potassium phosphate (2.0mmol) in a 25mL Schlenk reaction flask, in Under air, 5 mL of DMSO solvent was added, stirred at room temperature for 2 minutes, and reacted in an oil bath at 60° C. for 1.2 hours. After the reaction, the mixture was cooled to room temperature, filtered with diatomaceous earth, extracted with ethyl acetate and 10% ammonia water in mass concentration, collected the organic phase, dried over anhydrous magnesium sulfate, filtered, removed volatile components under reduced pressure, and then used a silica gel column to Chromatographic separation (eluent: petroleum ether (60-90°C) / ethyl acetate, v / v=10:1) gave the target product 1aa (159 mg, yield 90%) as a yellow liquid. The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 2
[0043]
[0044]Sequentially weigh α-carbonyl enamine 2a (0.5mmol), boron tetrafluoride p-bromobenzenediazonium salt 3b (0.6mmol), copper chloride (0.15mmol), potassium carbonate (1.5mmol) in a 25mL Schlenk reaction flask , under the air, add DMF solvent 5mL, stir at room temperature for 2 minutes, put into 80°C oil bath to react for 1.2 hours. After the reaction, the mixture was cooled to room temperature, filtered with diatomaceous earth, extracted with ethyl acetate and 10% ammonia water in mass concentration, collected the organic phase, dried over anhydrous magnesium sulfate, filtered, removed volatile components under reduced pressure, and then used a silica gel column to Chromatographic separation (eluent: petroleum ether (60-90°C) / ethyl acetate, v / v=10:1) gave the target product 1ab (140 mg, yield 65%) as a yellow liquid. The target product was confirmed by NMR and high-resolution mass spectrometry.
Embodiment 3
[0046]
[0047] Weigh successively α-carbonyl enamine 2a (0.5mmol), boron tetrafluoride p-chlorobenzene diazonium salt 3c (0.6mmol), copper acetate (0.1mmol), cesium carbonate (1.0mmol) in a 25mL Schlenk reaction flask, Under argon, 5 mL of DMF / DMSO mixed solvent was added, stirred at room temperature for 2 minutes, and reacted in an oil bath at 60° C. for 2.0 hours. After the reaction, the mixture was cooled to room temperature, filtered with diatomaceous earth, extracted with ethyl acetate and 10% ammonia water in mass concentration, collected the organic phase, dried over anhydrous magnesium sulfate, filtered, removed volatile components under reduced pressure, and then used a silica gel column to Chromatographic separation (eluent: petroleum ether (60-90°C) / ethyl acetate, v / v=10:1) gave the target product 1ac (130 mg, yield 67%) as a yellow liquid. The target product was confirmed by NMR and high-resolution mass spectrometry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com