Pentamethine cyanine dye and preparation method thereof
A technology of pentamethyl sichuan cyanine and cyanine dyes, applied in the field of fluorescent probes, can solve the problem of insufficient variety of pentamethyl sichuan cyanine dyes, etc., and achieve the effect of facilitating confirmation of dyeing effect and concise process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
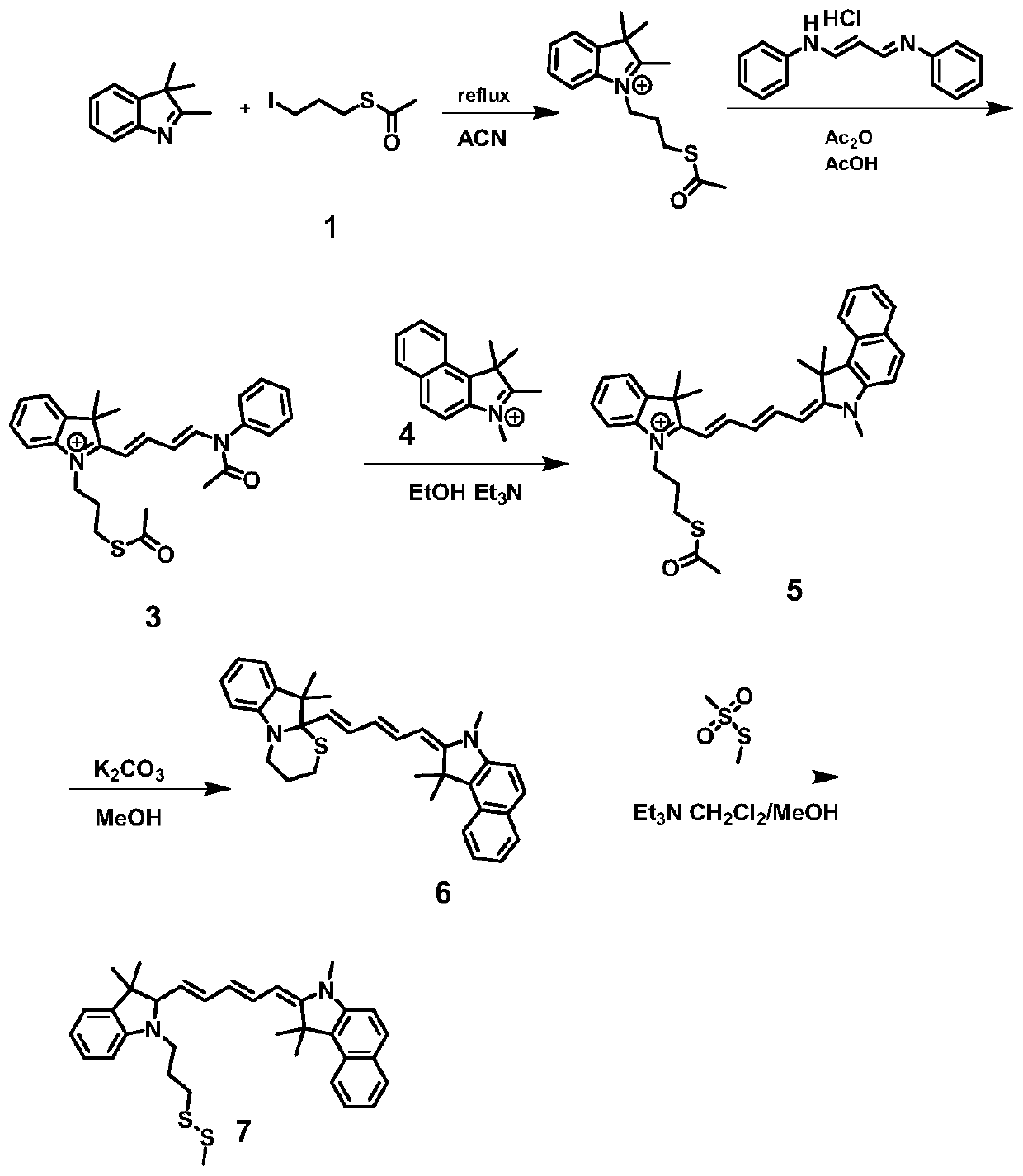
[0053] On the other hand, the present invention also provides a kind of preparation method of described pentamethine dye, comprises the steps:
[0054] S01: reacting alkyl halides containing substituents such as general formulas A, B and C with thioesters to obtain quaternary ammonium salts such as general formulas D, E and F;
[0055]
[0056] S02: the quaternary ammonium salt of the general formula D, E, F and malondialdehyde diphenylamine hydrochloride are heated and condensed under acid catalysis to generate intermediates such as the general formula G, H, and I;
[0057]
[0058] S03: the target product G, H, I of the condensation reaction is condensed with the compound of general formula A, B, C to obtain the thioester J, K, L, M of the cyanine dye;
[0059]
[0060] S04: deacylating the thioesters J, K, L, and M under alkaline conditions to generate intramolecular six-membered heterocyclic compounds N, O, P, and Q;
[0061]
[0062] S05: The six-membered het...
Embodiment 1
[0074] Embodiment 1 wherein a kind of preparation method of probe
[0075] Synthetic route such as figure 1
[0076] Specific steps are as follows:
[0077] Preparation of Compound 1
[0078] Add 1-bromo-3-chloropropane and potassium thioacetate solution (1:1) into THF to reflux for 1 hour, stir at room temperature for 24 hours, filter the inorganic salt, evaporate the organic phase to dryness and dissolve it with acetone, add an equivalent amount of NaI and stir at room temperature for 6 days , the organic phase was diluted with ethyl acetate, washed with sodium thiosulfate, dried and the organic phase was evaporated to dryness to obtain a pale yellow oil.
[0079] Preparation of Compound 2
[0080] Take 3.66g of 2,3,3-trimethyl-3H-indoline and 2.38g of compound 1 in 20ml of acetonitrile, add, under nitrogen atmosphere, heat to reflux for 6h, cool to room temperature, add diethyl ether to wash, pour the liquid organic phase , to obtain a purple oil (yield 40%).
[0081]...
Embodiment 2
[0092] Bioluminescent imaging detection of dyes
[0093] 1), the preparation of dye mother liquor
[0094] Weigh 1 mol of the dyes prepared in Examples 18-19 and place them in a brown glass bottle, add 100 mL of HPLC DMSO solvent, and mix well to obtain 1 mM dye mother liquor.
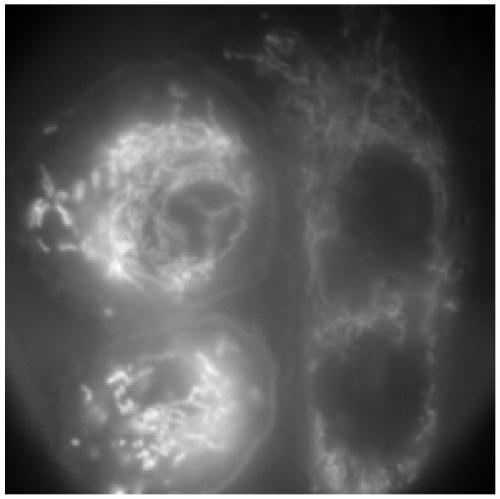
[0095] 2), HeLa cells were planted in a living cell culture dish, and after 48 hours, the medium was replaced, and the dye mother solution prepared in 1) was added to the culture dish so that the final concentration of the dye was 1uM, and placed in 30 Incubate at 7°C for 10 minutes, discard the culture medium, wash with PBS buffer 3 times, place on the laser confocal microscope stage, select a long-pass filter with an excitation wavelength of 633nm and an emission wavelength of 645nm for observation. Depend on figure 2 As shown, the dye can enter cells, and has different selectivity in cells, and can be applied to the application of live cell fluorescent dyes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com