Tumor ECM degrading and/or inhibiting agent and complete set kit and application thereof
A technology of inhibitors and tumors, which is applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., and can solve the problems of not being able to disperse and kill cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1. Sequence Design
[0086] The amino acid sequences involved in the embodiments of the present invention include Collagenase1, PH20, HAS2 (hyaluronidase synthesis gene). The NCBI accession number of the amino acid sequence of PH20 is NP_694859.1 (cDNA is NM_153189.2); the NCBI accession number of HAS2 is NP_005319.1 (cDNA is NM_005328.3).
[0087] The amino acid sequence information of the protein used in the present invention is shown in Table 1, and the base sequence encoding the amino acid of HAS2 is shown in Table 2.
[0088] Table 1. List of amino acid sequences
[0089]
[0090]
[0091] Table 2. Base sequence list
[0092]
[0093] Genewiz Company was commissioned to synthesize the HAS2 sequence.
Embodiment 2
[0094] Example 2. Vector Construction
[0095] The base sequence of HAS2 obtained in Example 1 was digested with NheI and BamHI, ligated with DNA Ligation Kit and inserted into the corresponding site of pcDNA3.1-Hyg vector (Invitrogen). The ligation products were transformed into competent Escherichia coli (Top10). On the second day after transformation, the clones were picked and cultured in LB liquid medium containing 100 μg / mL ampicillin (Saigon Biotechnology), and sequenced for identification. The clones with correct sequencing results were selected and inoculated into LB liquid medium containing 100 μg / mL ampicillin at a scale of 50 ml and cultured for about 16 hours. Bacteria were collected, and plasmids were extracted and purified using a plasmid extraction kit (Qiagen).
[0096] The resulting HAS2-pcDNA3.1-Hyg plasmid was linearized with FspI. The digested product was extracted with phenol-chloroform to obtain a preliminary linearized plasmid. The restriction endon...
Embodiment 3
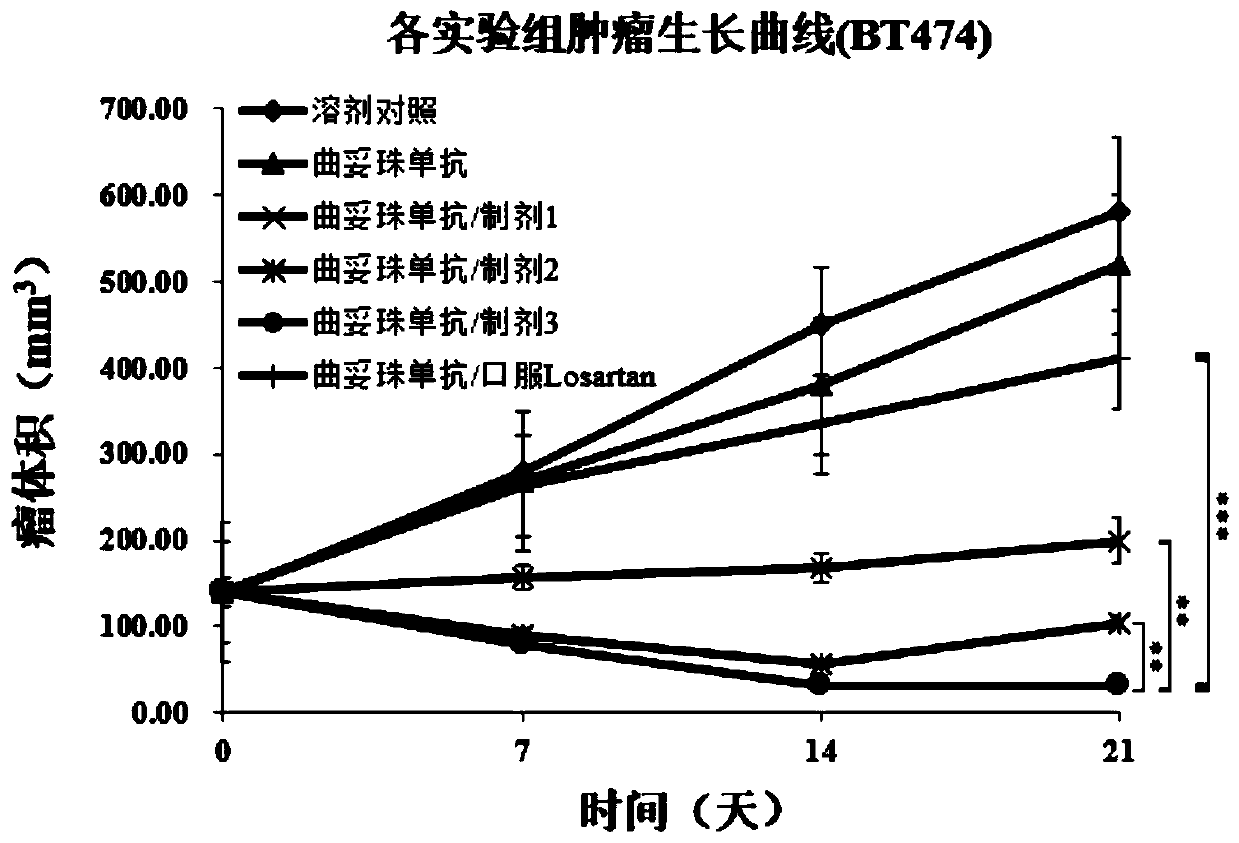
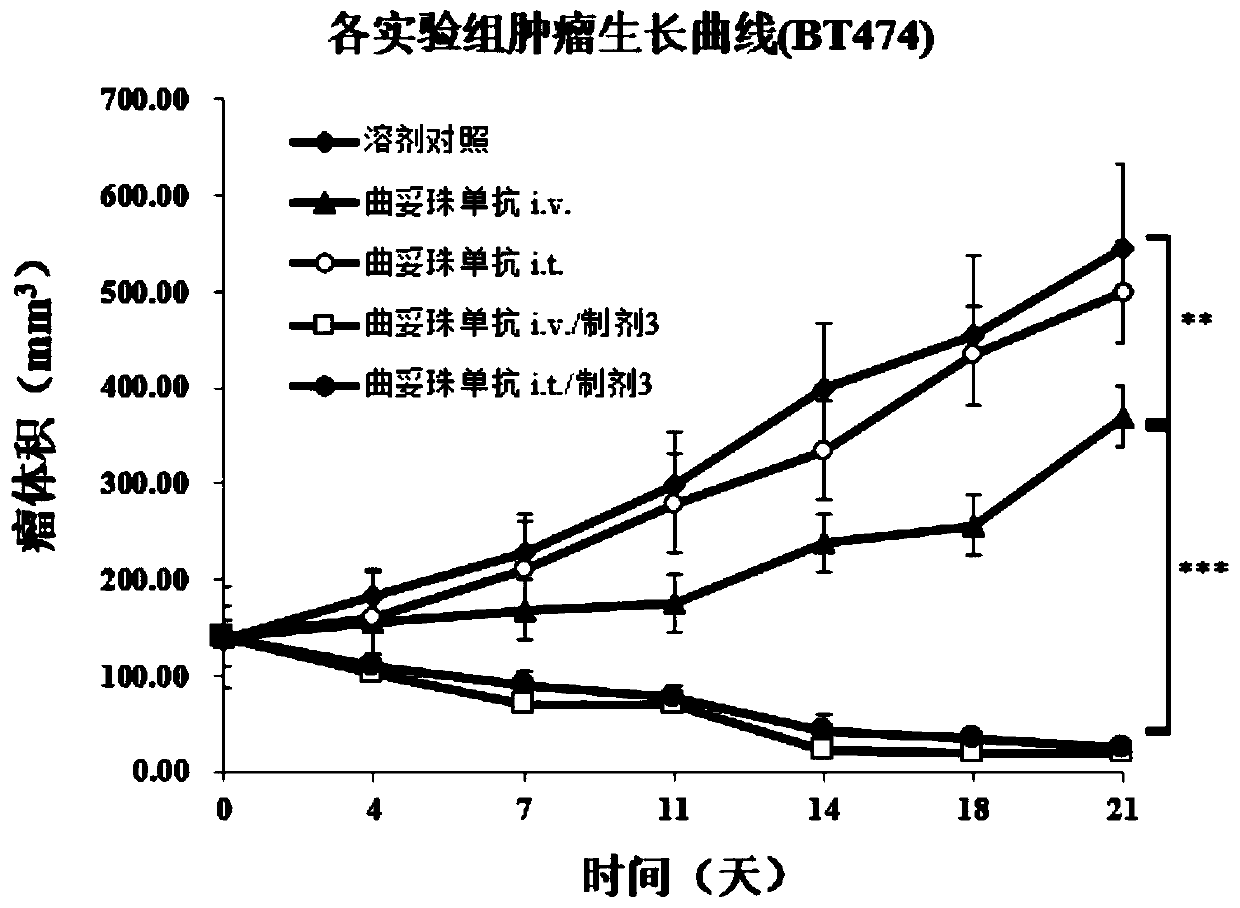
[0098] Embodiment 3. Preparation of preparation of the present invention
[0099] Different doses of hyaluronidase PH20 (Rhinobio), collagenase (Rhinobio), Losartan (Sigma) and buffer (disodium hydrogen phosphate), cryoprotectant (sucrose), excipient ( Mannitol), enzyme protection agent (which belongs to stabilizer, including disodium edetate, calcium chloride) and surfactant (Tween 20) are dissolved in water for injection, and the volume is adjusted to 6000mL. The preparation of 10,000 vials was divided into 0.6mL / vials for lyophilization.
[0100] The preparation can also be made into a solution, and the corresponding buffer (disodium hydrogen phosphate), stabilizer (sucrose, edetate disodium, calcium chloride) and surfactant (Tween 20) can be prepared according to Doses from Table 3 continued to be formulated.
[0101] Table 3. Tumor injection preparation formula
[0102]
[0103]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com