Volatile organic compound catalytic oxidation catalyst with high chlorine poisoning resisting efficiency, and preparation method thereof
A volatile organic compound, catalytic oxidation technology, applied in the direction of physical/chemical process catalyst, organic compound/hydride/coordination complex catalyst, metal/metal oxide/metal hydroxide catalyst, etc., can solve the problem of agglomeration Effectively solve problems such as strong CVOCs activation performance, maintain activity, and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
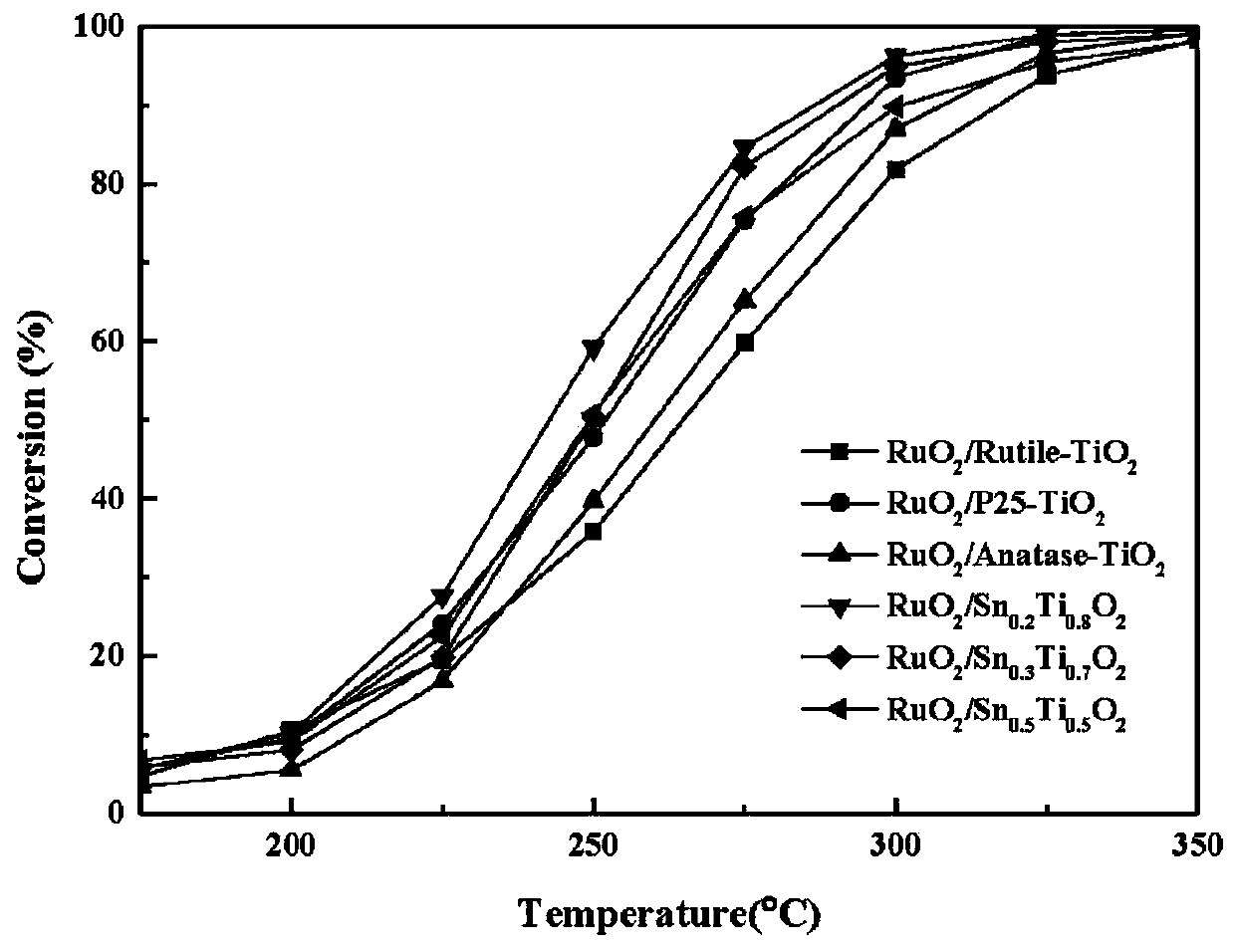
[0045] CVOCs Catalytic Oxidation Catalyst RuO 2 / Sn y Ti 1-y o 2 preparation of
[0046] Dissolve an appropriate amount of tin chloride in deionized water and stir for 30 minutes until completely dissolved. Then, according to the different doping ratios of Sn and Ti, the corresponding chemical equivalent of tetrabutyl titanate was slowly added dropwise to the aqueous solution of tin chloride (Sn y Ti 1-y o 2 In the preparation process of the method, the molar ratio of tin chloride and tetrabutyl titanate used is y:1-y), the dropping process is carried out with vigorous stirring, and after the dropping is completed, continue to stir vigorously for 30 minutes to ensure that the solution is fully mixed . Finally, ammonia water was added dropwise into the obtained mixed solution at a rate of 60 drops per minute until the pH value of the solution was 10. After suction filtration, the obtained sediment was aged for 12 hours and then washed three times with deionized water an...
Embodiment 2
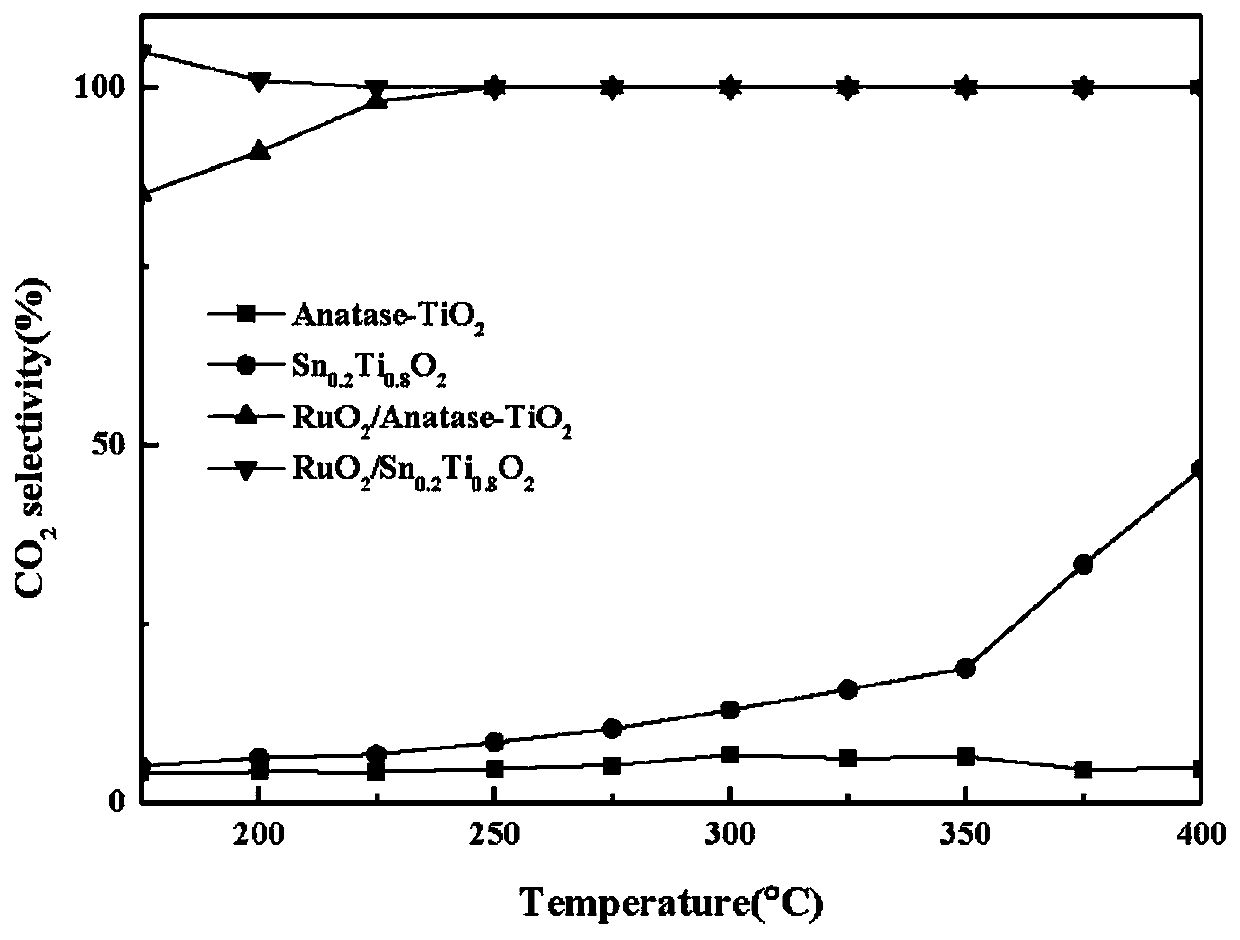
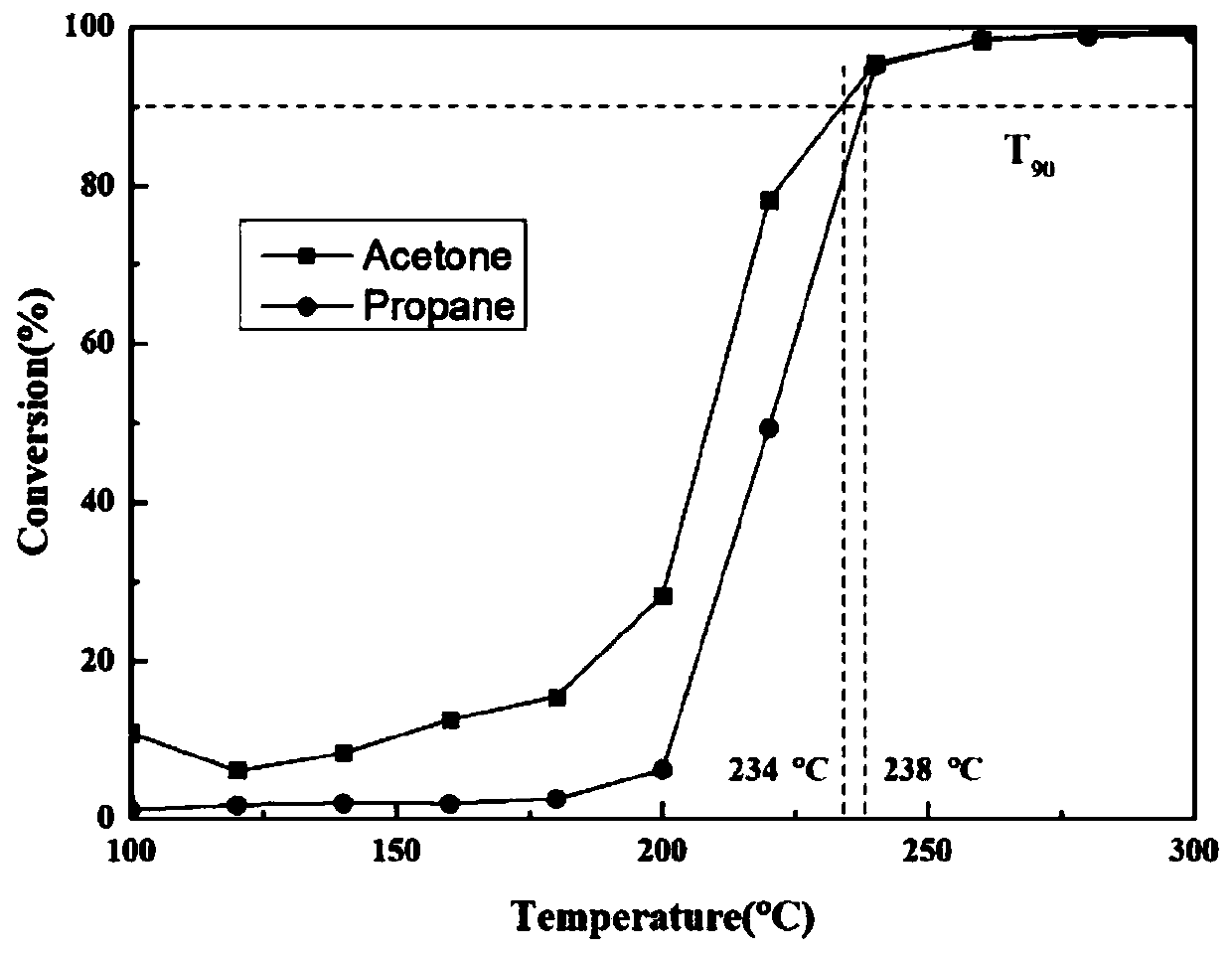
[0051] Get the RuO described in embodiment 1 2 / Sn 0.2 Ti 0.8 o 2 Catalytic oxidation catalyst 200mg, the test conditions are: 1000ppm acetone, 20% O 2 , N 2 Equilibrium, 30000 / h space velocity, through gasmet to its catalytic activity and CO 2 Selectively tested, activity results see image 3 .
[0052] Get the RuO described in embodiment 1 2 / Sn 0.2 Ti 0.8 o 2 Catalytic oxidation catalyst 200mg, test conditions: 1000ppm propane, 20% O 2 , N 2 Equilibrium, 30000 / h space velocity, test its catalytic activity by gasmet, see the activity result image 3 .
[0053] From image 3 It can be seen that the high-efficiency chlorine-resistant CVOCs catalytic oxidation catalyst in the present invention also has high catalytic activity for the catalytic oxidation of various VOCs such as acetone and propane. When the temperature is lower than 300 ° C, both acetone and propane have been completely converted.
Embodiment 3
[0055] Get the CVOCs catalytic oxidation catalyst described in embodiment 1 (RuO 2 / Sn 0.2 Ti 0.8 o 2 ) 200mg, the test conditions are: 1000ppm dichloromethane, 20%O 2 , N 2 Equilibrium, 30000 / h space velocity, stability test is carried out under the condition of temperature of 250 ℃, the stability test results are shown in Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com