Polypeptide, polypeptide nano drug-loading carrier and preparation method of polypeptide and polypeptide nano drug-loading carrier
A nano-drug loading and carrier technology, applied in the field of bioengineering, can solve problems such as unsatisfactory splitting conditions, and achieve the effects of avoiding potential biological toxicity and immunogenicity, low synthesis cost and good stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: Preparation of Ac-LLLLLLKKKK-NH2 polypeptide
[0060] Step 1. Distill N,N-dimethylformamide (DMF) and piperidine (Piperidine) solvents
[0061] Distill the purchased DMF solution under reduced pressure at 60°C to obtain pure DMF solvent; add a small amount of CaH2 to the purchased piperidine and heat to reflux for 1-2 hours, and receive the fraction with boiling point temperature (106°C) to obtain pure piperidine pyridine solvent.
[0062] Step 2, preparation of amino acid, resin, activator, capping agent, deprotecting agent
[0063] The amount of amino acids and other reagents required to prepare 0.25mM NH2-AAAAAAKK-NH2 is calculated on the polypeptide solid-phase synthesizer:
[0064] Leu (leucine): 1.97g dissolved in 21mL DMF;
[0065] Lys (lysine): 2.27g dissolved in 32mL DMF;
[0066] Resin (loaded at 0.6mmol / g): 0.417g;
[0067] Washing: TFA: 142mL;
[0068] Deprotection solution: piperidine: 48mL; DMF: 192mL (20% piperidine / 80% DMF);
[0069]...
Embodiment 2
[0075] Example 2: Preparation of Ac-LLLLLLKKKK-NH2 drug-loaded carrier
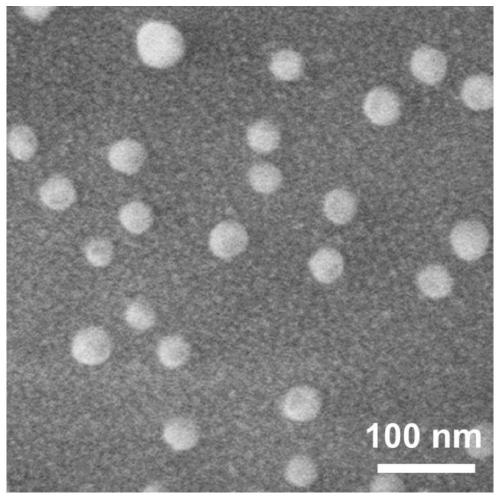
[0076] Weigh 1.25mg (1mmol / L) polypeptide Ac-LLLLLLKKKK-NH2, add 1mL of Hepes buffer solution, sonicate for 5min, and let it stand at room temperature for 1h or 6 months. SEM and TEM observation results show that stable nanoparticles are formed at this time. Assembly ( figure 1 , Figure 4 ).
Embodiment 3
[0077] Example 3: Detection of self-assembled morphology of Ac-LLLLLLKKKK-NH2 drug-loaded carrier in Hepes buffer
[0078] The specific detection method is as follows:
[0079] Examination of self-assembled morphologies (TEM, SEM) in Hepes buffer.
[0080] SEM: The prepared peptide solution was lyophilized into powder using a vacuum freeze dryer (EYELAFDU-1100, Japan) to obtain sample powder. On a scanning electron microscope (SEM, HITACHI, SU8010, Japan), the sample was observed at a voltage of 3 kV after spraying gold. The results showed that the peptide sample Ac-LLLLLLKKKK-NH2 observed by scanning transmission electron microscopy self-assembled into a uniform nanosphere structure in Hepes buffer, as Figure 5 shown.
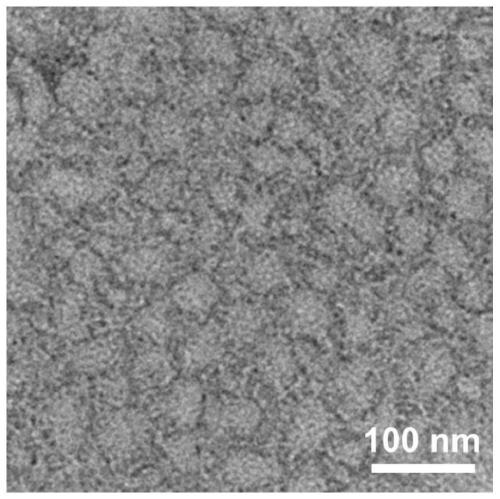
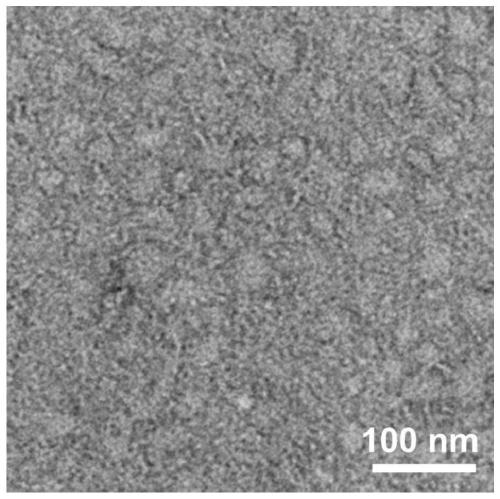
[0081] TEM: Transmission electron microscopy (TEM) measurements were performed on a HITACHI HT7700 instrument (Japan) at an accelerating voltage of 80 kV. Take the peptide solution obtained in Example 2 and let it stand for a specific time, or adjust a sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com