Method for detecting pathogenic bacteria of listeria monocytogenes from clinical blood
A mononuclear cell hyperplasia and Listeria monocytogenes technology is applied in the field of detection of pathogenic bacteria of blood infection, which can solve the problems of low specificity, long detection period and low sensitivity, and achieve the effects of high specificity, high sensitivity and improved detection efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: PCR primer design
[0039] Utilize Listeria monocytogenes genomic DNA sequence to design a specific PCR primer, this specific PCR primer comprises the forward primer shown in SEQ ID NO.1 and the reverse primer shown in SEQ ID NO.2 ;in,
[0040] SEQ ID NO.1: 5'-GGGAAATCTGTCTCAGGTGATGT-3';
[0041] SEQ ID NO.2: 5'-CGATGATTTGAACTTCATCTTTTGC-3';
[0042] The length of the target fragment amplified by the primers is 106bp.
Embodiment 2
[0044] Listeria monocytogenes-specific primers were used to detect pathogenic bacteria such as Listeria monocytogenes, Staphylococcus aureus, Salmonella, Pseudomonas aeruginosa, Klebsiella pneumoniae and Escherichia coli added in blood. In this example, OD is added to every 100 μL of blood 600 0.1 of the above-mentioned various pathogenic bacteria. This example includes the extraction of each bacterial genome DNA in the blood and the PCR amplification detection of the specific gene of Klebsiella pneumoniae. This method comprises the following steps:
[0045] (1) Extraction of genomic DNA of Listeria monocytogenes in blood
[0046] (1) Collect 100 μL cultured OD 600 =0.1 for Listeria monocytogenes, Staphylococcus aureus, Salmonella, Pseudomonas aeruginosa, Klebsiella pneumoniae and Escherichia coli. Centrifuge at 12000 rpm for 1 minute to collect the precipitated bacteria.
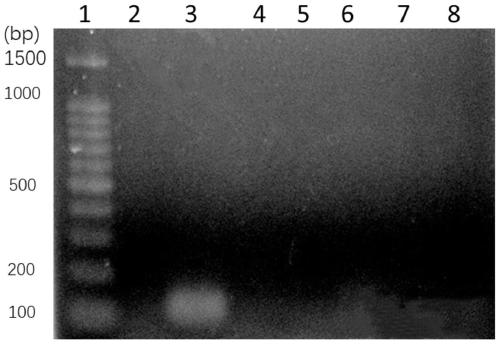
[0047] (2) Take 100 μL of fresh blood from 6 groups, and add Listeria monocytogenes, Staphylococcus a...
Embodiment 3
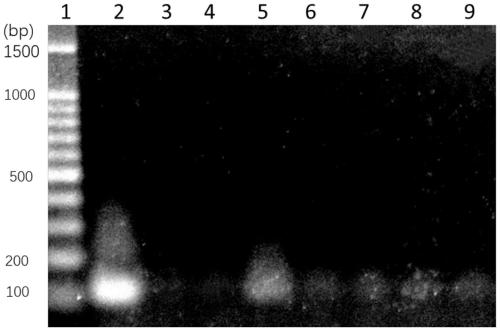
[0064] 100 μL of Listeria monocytogenes-specific primers were used to detect Listeria monocytogenes added to the blood. In this example, 10 9 , 10 7 , 10 5 , 10 3 , 10CFU of Listeria monocytogenes. This example includes the extraction and PCR amplification detection of bacterial genomic DNA in blood. This method comprises the following steps:
[0065] (1) Collect 10 cultivated 9 , 10 7 , 10 5 , 10 3 , 10 CFU of Listeria monocytogenes. Centrifuge at 12000 rpm for 1 minute to collect the precipitated bacteria.
[0066] (2) Take 5 groups of 100 μL of fresh blood, add the different numbers of Listeria monocytogenes precipitated in step (1) to the 5 groups of 100 μL of fresh blood, resuspend the precipitated cells, and pipette fully well mixed.
[0067] (3) Add 100 μL enzyme lysate solution to each group of blood, and incubate at 37° C. for 15 minutes. The enzyme lysate contains: 0.1 mg / mL ribonuclease A, 1 mg / mL proteinase K, 0.05 mg / mL broad-spectrum lyase ClyH, 10 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com