Decarboxylation and Decarboxylation Radical Addition of Alkyl Carboxylates of Photoactivated Electron Donor-Acceptor Complexes
An electron donor and addition reaction technology, which is applied in the field of decarboxylation of alkyl carboxylate and decarboxylation Giese radical addition reaction, can solve the problems of reducing economic benefits of production, hidden dangers of product safety, and increasing raw material costs, so as to reduce expensive Raw material input, the effect of improving purity and safety, and reducing the output of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 Free radical clock experiment
[0054] 1. Main medicines needed
[0055] Dichloromethane (DCM), N,N-dimethylacetamide (DMA).
[0056] 2. Data collection
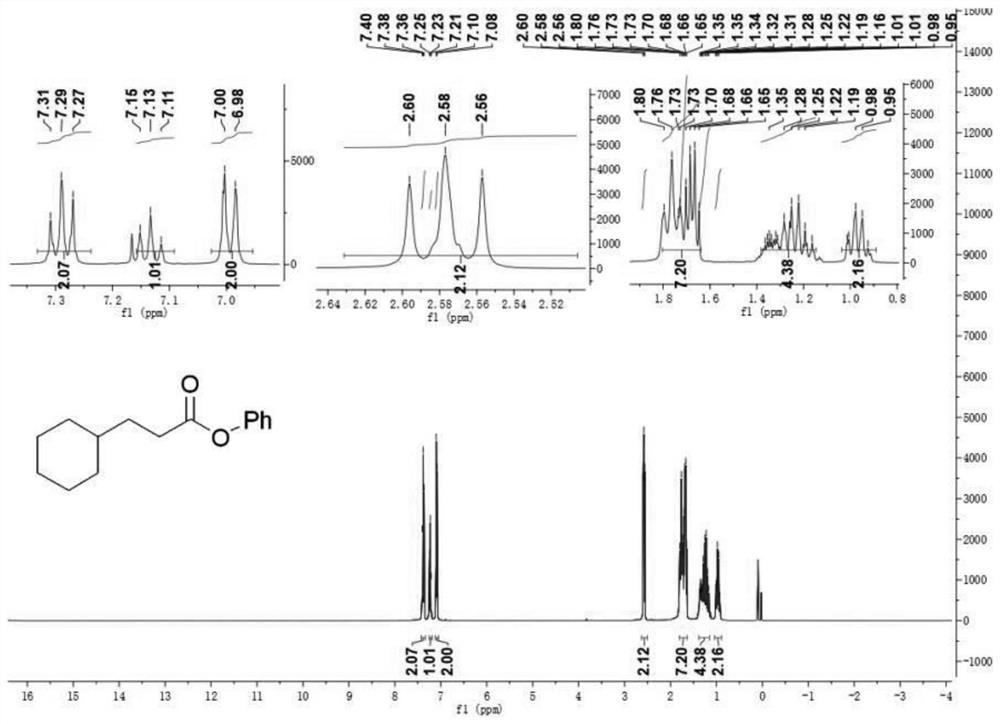
[0057] Thin Layer Chromatography (TLC), Gas Chromatography-Mass Spectrometry (GC-MS), Nuclear Magnetic Resonance Spectrometry (NMR)
[0058] 3. Reaction steps of the free radical clock experiment
[0059] 3.1 Drop into a Schlenk tube equipped with a stirrer (1.0 equiv, 0.2 mmol), (0.3 mmol), HE (0.3 mmol), tetramethylpiperidine nitroxide (TEMPO) (2.0 equiv, 0.4 mmol), evacuated the original gas in the tube and passed argon to fill the tube space (repeat 3 times) . Under an argon atmosphere, anhydrous DMA (2.0 mL) was injected into the reaction system. The reaction system was illuminated with 40 W of blue (456 nm) LED illumination at room temperature while stirring the mixture in the reaction system continuously for 12 h. The resulting mixture was quenched with saturated NaCl solution and suction fi...
Embodiment 2
[0066] Example 2 Gram-scale experiment
[0067] In this example, the experiment carried out in Section 3.2 of Example 1 is scaled up to a gram-scale experiment. Drop into Schlenk tube with stirrer (6 mmol), (9 mmol), HE (9 mmol), evacuated the original gas in the tube and passed argon to fill the tube space (repeated 3 times). Under an argon atmosphere, anhydrous DMA (50 mL) was injected into the reaction system. The reaction system was illuminated with 40 W of blue (456 nm) LED illumination at room temperature while stirring the mixture in the reaction system continuously for 12 h. After the reaction, the obtained mixture was quenched with saturated NaCl solution, and then subjected to suction filtration with 50 mL of ethyl acetate, and the suction filtration was repeated 3 times. The product was separated by flash column chromatography on a silica gel plate, and the eluent used was: petroleum ether / ethyl acetate=10 / 1.
[0068] The reactant reaction conditions and the ...
Embodiment 3
[0072] 1. Experiment setup method
[0073] 1.1 The HE and RAE (ie, ) according to the reaction concentration in Example 1 to prepare the DMA solution of HE, the DMA solution of RAE and the DMA solution of HE+RAE (c=c*), and take part of the DMA solution of HE+RAE (c=c*) to dilute 10 times to obtain a DMA solution of HE+RAE (c=0.1c*).
[0074] 1.2 The HE and RAE (ie, ) according to the reaction concentration in Example 1 to prepare HE DCM solution, RAE DCM solution and HE+RAE DCM solution (c=c*), and take part of HE+RAE DCM solution (c=c*) to dilute 10 times to obtain HE+RAE in DCM (c=0.1 c*).
[0075] 2. Data acquisition method
[0076] The absorption spectrum of the above solution was tested using a UV-Vis absorption spectrometer.
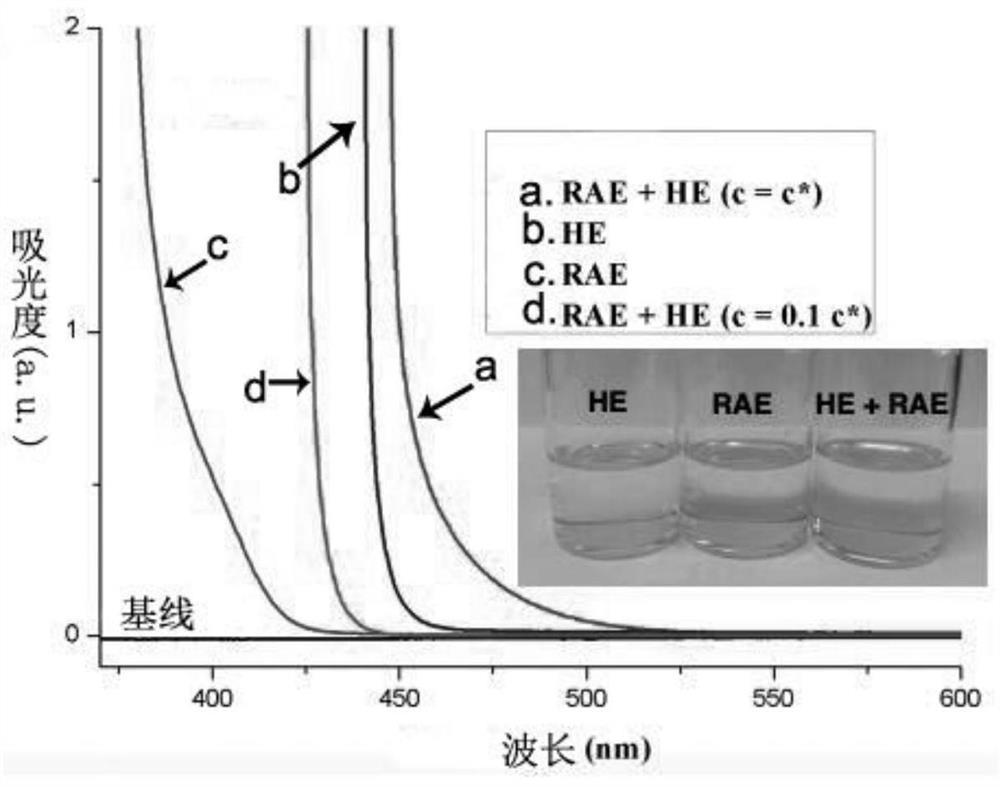
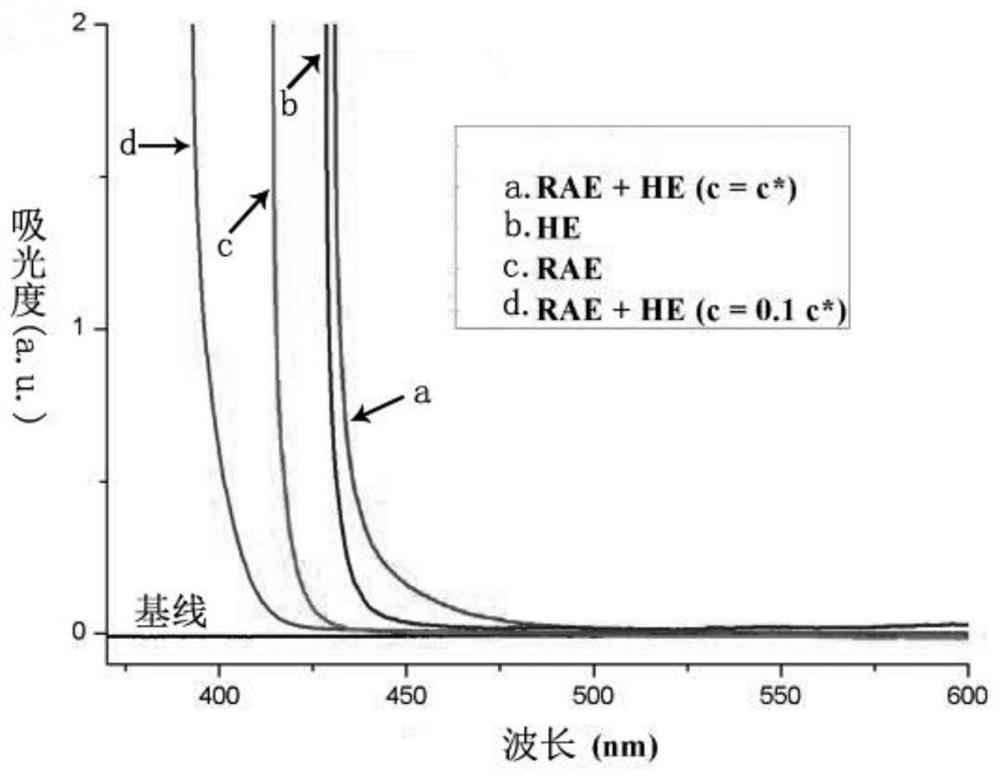
[0077] 3. Test results
[0078] Test results such as figure 1 and figure 2 shown. like figure 1 As shown, the absorption spectrum peaks of HE+RAE in DMA solution (c=c*) with HE and RAE dissolved in DMA simultaneously to simulate the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com