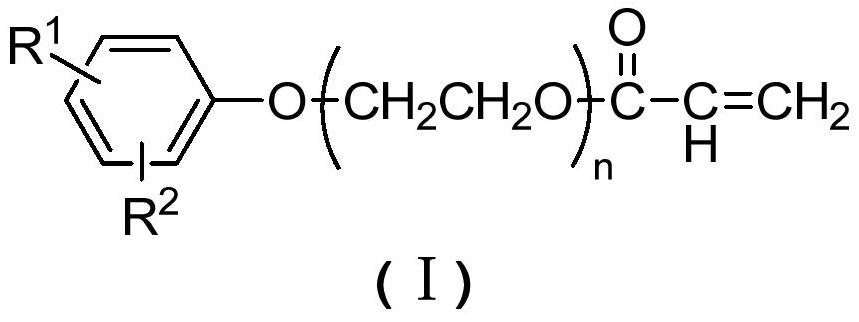A kind of polymerizable surfactant containing aromatic hydrocarbon structure and preparation method thereof
A technology for surfactants and aromatic hydrocarbon structures, applied in the field of polymerizable surfactants containing aromatic hydrocarbon structures and its preparation, can solve the problems of not providing a preparation method for polymerizable surfactants, achieve high yield, simple treatment process, Store convenient effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1 (method one)
[0069] (1) Add 3.0000g (6.383mmol) di-tert-butylphenoxy polyoxyethylene ether (n=6 ) and 40mL of toluene were stirred until completely dissolved, then added 0.0600g (0.545mmol) hydroquinone, 0.0572g (0.332mmol) p-toluenesulfonic acid, stirred until dissolved, finally added 0.8100g (11.241mmol) acrylic acid, warming up to 120 ℃ and react at this temperature for 6 h, and separate the water generated by the reaction.
[0070] (2) Pour the reaction solution in step (1) into a separatory funnel, wash several times with 5wt% aqueous sodium bicarbonate solution and saturated aqueous sodium chloride solution respectively, remove the solvent from the obtained organic phase and then vacuum dry for 24h to obtain a pale yellow oily compound, which is di-tert-butylphenoxy polyoxyethylene ether acrylate (DPP) containing 6 ethyleneoxy EO units 6 AA) 2.85 g.
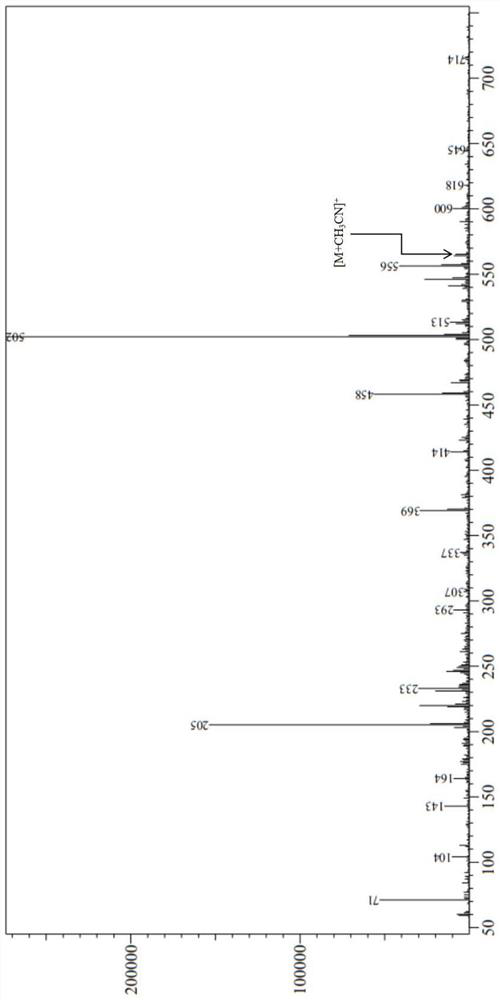
[0071] The liquid chromatography-mass spectrometry (LC-MS) diagram of the pale yellow oil prepar...
Embodiment 2
[0074] Embodiment 2 (method one)
[0075] The preparation method is the same as in Example 1, except that: in step (1), 5.0000g (14.793mmol) of di-tert-butylphenoxy polyoxyethylene ether (n=3) containing 3 ethyleneoxy EO units are added ), 0.1200 g (1.090 mmol) hydroquinone, 0.1366 g (0.793 mmol) p-toluenesulfonic acid and 1.8300 g (25.396 mmol) acrylic acid.
[0076] The obtained yellow oil is di-tert-butylphenoxy polyoxyethylene ether acrylate (DPP) containing 3 ethyleneoxy EO units. 3 AA) 4.7 g.
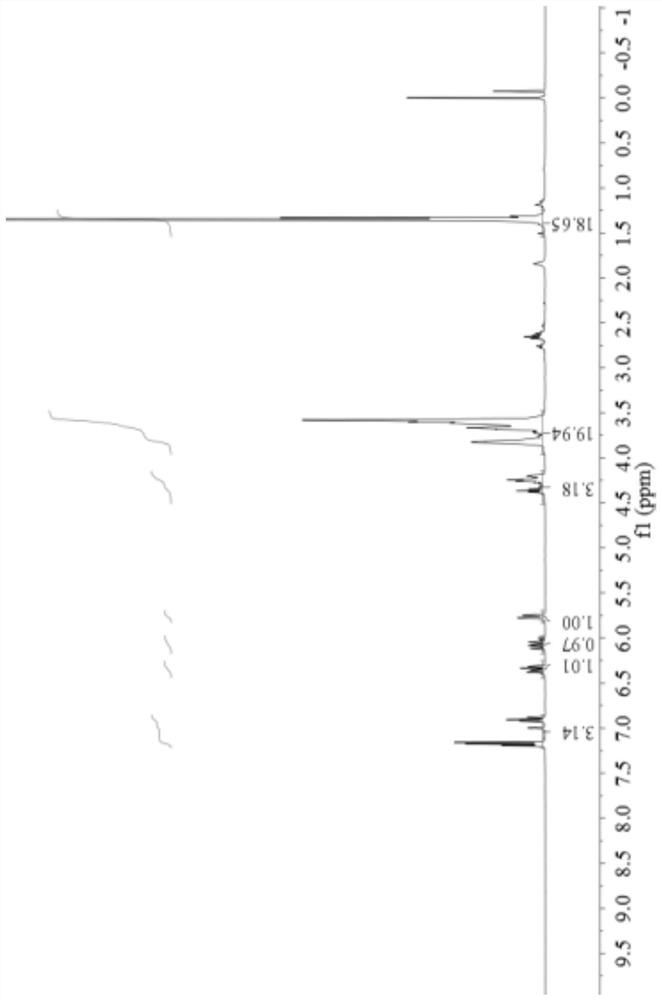
[0077] Its H NMR data are as follows:
[0078] 1 H NMR (400MHz, CDCl 3 ): δ(ppm) 6.9-7.3 (3H, hydrogen on benzene ring), 6.39 (1H, hydrogen on double bond C=CH 2 ), 6.13 (1H, hydrogen on double bond C=CH-CO), 5.81 (1H, hydrogen on double bond C=CH 2 ),3.0-4.5(12H, is 3-OCH 2 CH 2 O-), 0.5-2.2 (18H, which is tert-butyl hydrogen).
[0079] The obtained product was confirmed to be di-tert-butylphenoxy polyoxyethylene ether acrylate (DPP) containing 3 ethyleneoxy EO units thr...
Embodiment 3
[0080] Embodiment 3 (method one)
[0081] The preparation method is the same as in Implementation 1, except that: in step (1), 5.0000g (8.993mmol) of 3-ethyl-5-nonylphenoxypolyoxyethylene containing 7 ethyleneoxy EO units are added Ether (n=7), 0.0842 g (0.7647 mmol) hydroquinone, 0.0693 g (0.402 mmol) p-toluenesulfonic acid, 1.2961 g (17.986 mmol) acrylic acid.
[0082] The obtained yellow oil was 4.83 g of 3-ethyl-5-nonylphenoxy polyoxyethylene ether acrylate containing 7 ethyleneoxy EO units.
[0083] Its H NMR data are as follows:
[0084] 1 H NMR (400MHz, CDCl 3 ): δ(ppm) 6.9-7.3 (3H, hydrogen on benzene ring), 6.39 (1H, hydrogen on double bond C=CH 2 ), 6.13 (1H, hydrogen on double bond C=CH-CO), 5.81 (1H, hydrogen on double bond C=CH 2 ),3.0-4.5(28H, is 7-OCH 2 CH 2 O-), 0.5-2.2 (24H, is the alkyl hydrogen on the benzene ring).
[0085] The obtained product was confirmed to be 3-ethyl-5-nonylphenoxy polyoxyethylene ether acrylate containing 7 ethyleneoxy EO unit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com