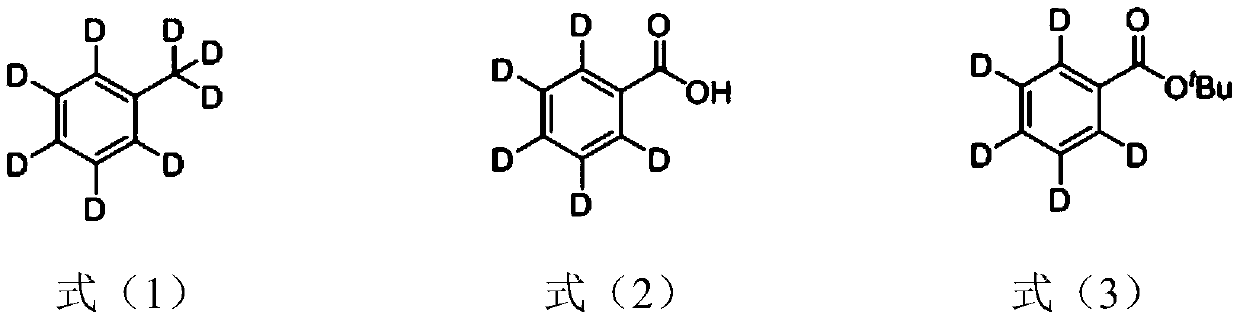
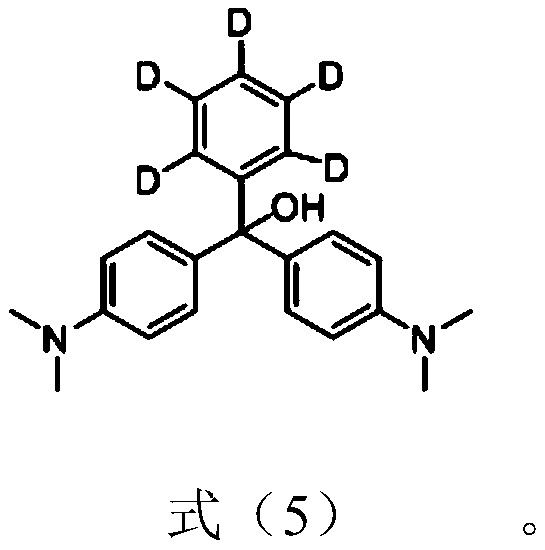
Synthetic method of pentadeuterium-substituted malachite green salt
A synthesis method, malachite green technology, applied in the direction of organic chemistry methods, chemical instruments and methods, carboxylate preparation, etc., can solve problems such as unsuitable synthesis of malachite green salt, harsh conditions, unsuitable synthesis methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of pentadeuterium-substituted leuco malachite green
[0056] Step a): preparation of pentadeuterium substituted tert-butyl benzoate;
[0057] Add octadeuterium-substituted toluene (3.5mL, 33mmol), potassium permanganate (12.01g, 76mmol), sodium carbonate (1.48g, 14mmol) and 100mL of water into a 250mL flask, reflux for 8h and cool to room temperature . After filtering with diatomaceous earth, it is acidified with hydrochloric acid with a concentration of 4mol / L, and then extracted three times with dichloromethane. After washing with water, the combined organic phase is dried with anhydrous sodium sulfate. After filtering, the solvent is removed under reduced pressure to obtain the compound shown in formula (2). Deuterium-substituted benzoic acid;
[0058] Take 50 mL of the obtained pentadeuterium-substituted benzoic acid, add 1 mL of concentrated sulfuric acid, tert-butanol (3.2 mL, 35 mmol), and 1 g of magnesium sulfate, reflux for 2 h and cool to room te...
Embodiment 2
[0074] Preparation of pentadeuterium-substituted malachite green tartrate
[0075] The pentadeuterium-substituted leuco malachite green crude product obtained in Example 1 (5.03 g, 15 mmol), 100 mL of methanol, and 10 mL of tartaric acid aqueous solution with a concentration of 1 mol / L were added to a 250 mL flask, and refluxed for 1 h. After cooling, let the liquid separation separate the organic phase and the water phase; get the water phase after the liquid separation and extract it three times with ether; combine the organic phase obtained by the liquid separation and the organic phase obtained by the extraction, and wash the combined organic phase with water, Then dry it with anhydrous sodium sulfate, filter and remove the solvent under reduced pressure; the obtained crude product is chromatographed by methanol / chloroform column to obtain 6.88 g of pentadeuterium-substituted malachite green tartrate, with a yield of 51%.
[0076] The H NMR spectrum data of malachite green...
Embodiment 3
[0081] Preparation of pentadeuterium-substituted malachite green chloride
[0082] Malachite green chloride, the chloride salt of malachite green.
[0083] The pentadeuterium-substituted leuco malachite green crude product obtained in Example 1 (5.03 g, 15 mmol), 100 mL of methanol, and 1 mL of concentrated hydrochloric acid (concentration: 12 mol / L) were added to a 250 mL flask, and refluxed for 1 h. After cooling, let the liquid separation separate the organic phase and the water phase; get the water phase after the liquid separation and extract it three times with ether; combine the organic phase obtained by the liquid separation and the organic phase obtained by the extraction, and wash the combined organic phase with water, Then dry it with anhydrous sodium sulfate, filter and remove the solvent under reduced pressure; the obtained crude product is chromatographed on a methanol / chloroform column to obtain 2.47 g of pentadeuterium-substituted malachite green chloride, with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com