Fluazinam hapten, fluazinam artificial antigen, fluazinam antibody, preparation methods of hapten and artificial antigen, and application of antibody
A technology of artificial antigen and fluazinam, which is applied in the preparation methods of peptides, chemical instruments and methods, animal/human proteins, etc., to achieve the effects of enhanced immunogenicity, easy availability of raw materials, and simple reaction operations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
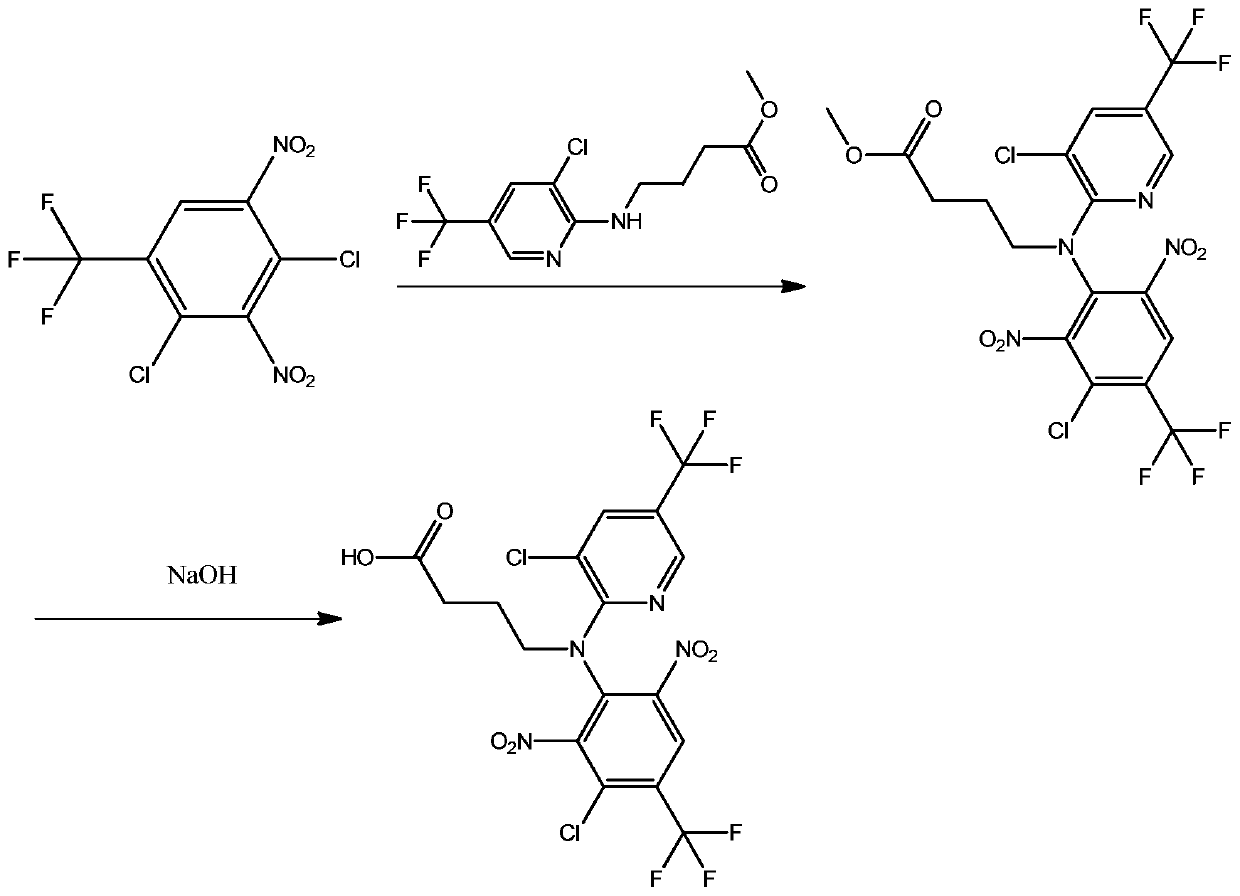
[0029] In a second aspect, the present invention provides a method for preparing the above-mentioned fluazinam hapten, which comprises the following steps:
[0030] 1) 2,4-dichloro-3,5-dinitrobenzotrifluoride reacts with 2-aminobutyric acid methyl ester-3-chloro-5-trifluoromethylpyridine to obtain intermediate 1, the intermediate Body 1 has the formula
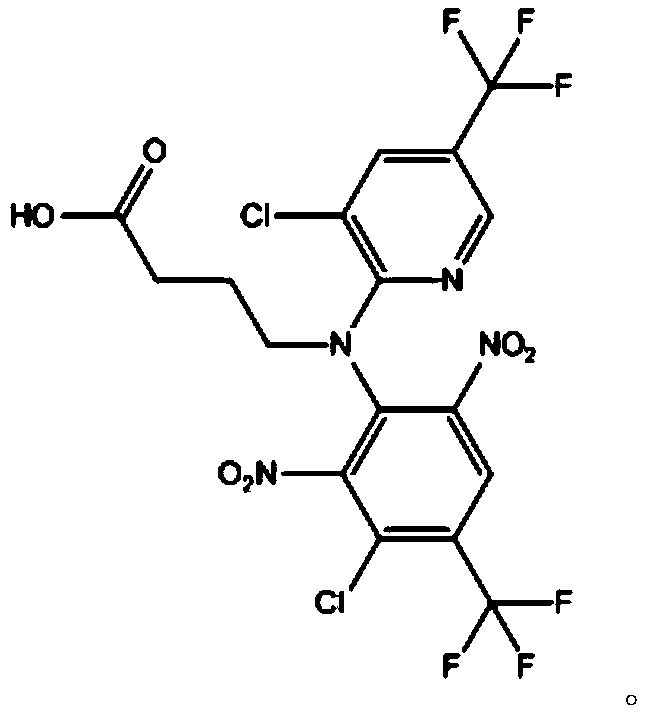
[0031] 2) The intermediate 1 is hydrolyzed to obtain the fluazinam hapten.
[0032] Preferably, the step 1) includes the following steps: dissolving 2,4-dichloro-3,5-dinitrobenzotrifluoride in an organic solvent, adding anhydrous potassium carbonate and methyl 2-aminobutyrate-3 -Chloro-5-trifluoromethylpyridine, oil bath heating reaction, after the reaction, rotary steaming, adding water, extracting with ethyl acetate, collecting the organic phase, after the organic phase was evaporated to dryness, dichloromethane-cyclohexane 2,4-dichloro-3,5-dinitrotrifluorotoluene, anhydrous potassium carbonate and 2-aminobutyric acid me...
Embodiment 1
[0051] A method for preparing a fluazinam hapten, comprising the steps of:
[0052] 1) Dissolve 0.304g of 2,4-dichloro-3,5-dinitrobenzotrifluoride in 80mL of acetonitrile, add 0.32g of anhydrous potassium carbonate and 0.21g of methyl 2-aminobutyrate-3-chloro-5 -Trifluoromethylpyridine, heated in an oil bath for 12 hours. After the reaction, acetonitrile was removed by rotary evaporation, 60 mL of water was added, extracted with 80 mL of ethyl acetate, and the organic phase was collected. : 3 dichloromethane-hexanaphthene beating, suction filtration, washing with n-hexane to obtain intermediate 1;
[0053] 2) Weigh intermediate 1, add alkaline solution, heat to reflux, and carry out hydrolysis reaction; after the reaction, adjust the pH to slightly acidic with hydrochloric acid, then extract with ethyl acetate, and obtain the obtained product after the organic phase is evaporated to dryness and purified by column Describe fluazinam hapten; Wherein, described alkaline solution...
Embodiment 2
[0055] A method for preparing a fluazinam hapten, comprising the steps of:
[0056] 1) Dissolve 0.304g of 2,4-dichloro-3,5-dinitrobenzotrifluoride in 80mL of acetonitrile, add 0.28g of anhydrous potassium carbonate and 0.17g of methyl 2-aminobutyrate-3-chloro-5 -Trifluoromethylpyridine, heated in an oil bath for 12 hours. After the reaction, acetonitrile was removed by rotary evaporation, 60 mL of water was added, extracted with 80 mL of ethyl acetate, and the organic phase was collected. : 3 dichloromethane-hexanaphthene beating, suction filtration, washing with n-hexane to obtain intermediate 1;
[0057] 2) Weigh intermediate 1, add alkaline solution, heat to reflux, and carry out hydrolysis reaction; after the reaction, adjust the pH to slightly acidic with hydrochloric acid, then extract with ethyl acetate, and obtain the obtained product after the organic phase is evaporated to dryness and purified by column Describe fluazinam hapten; Wherein, described alkaline solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com