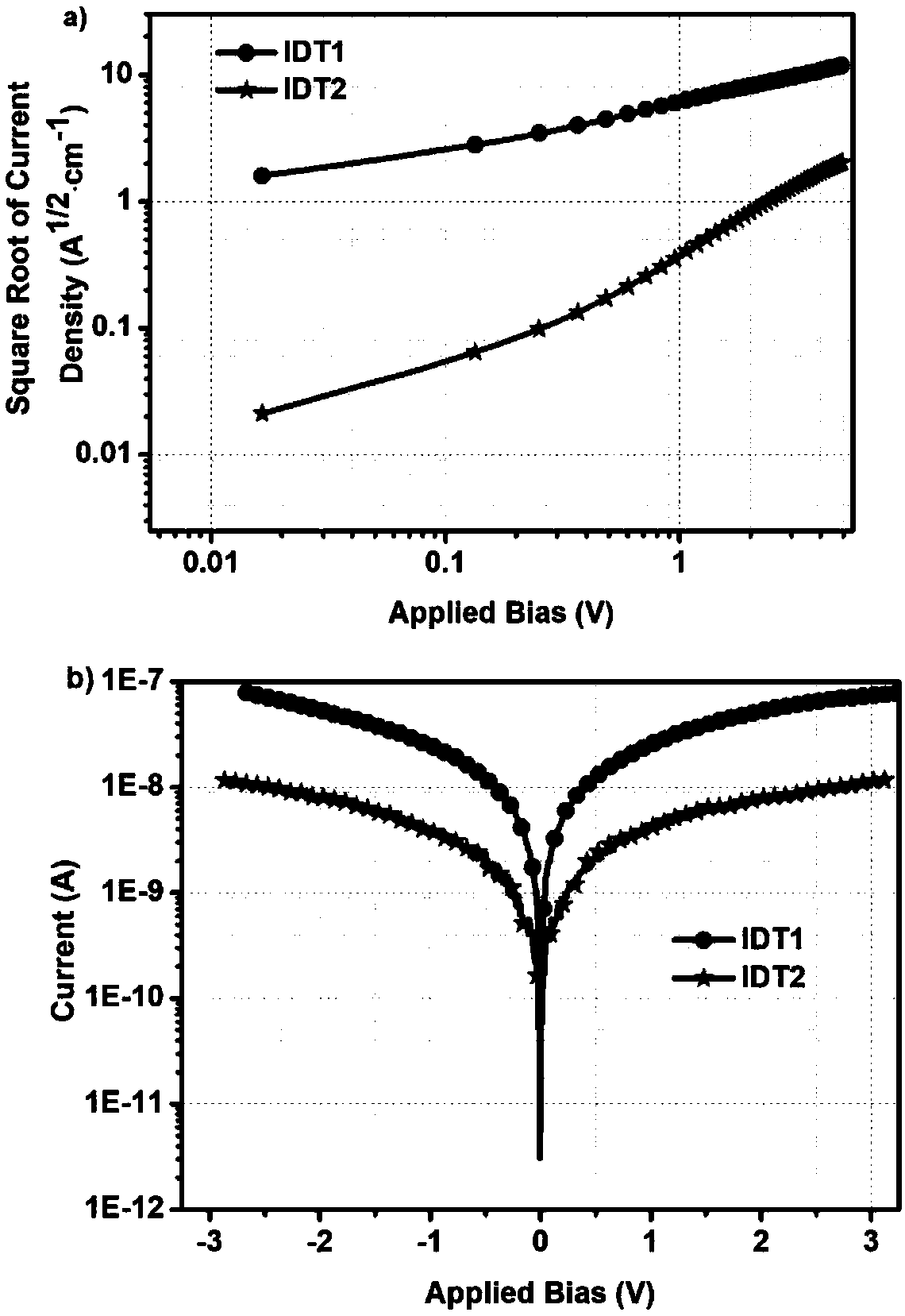
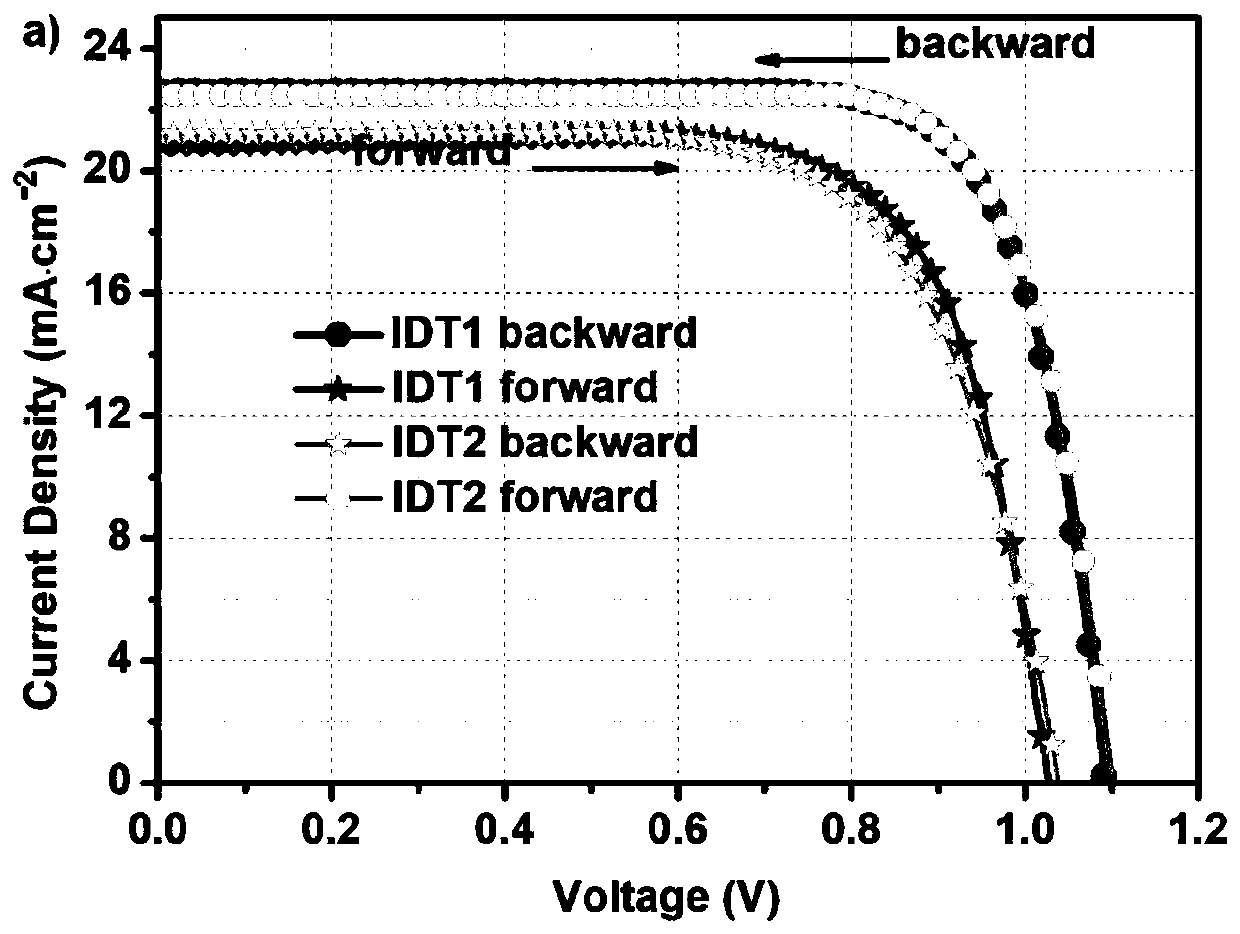
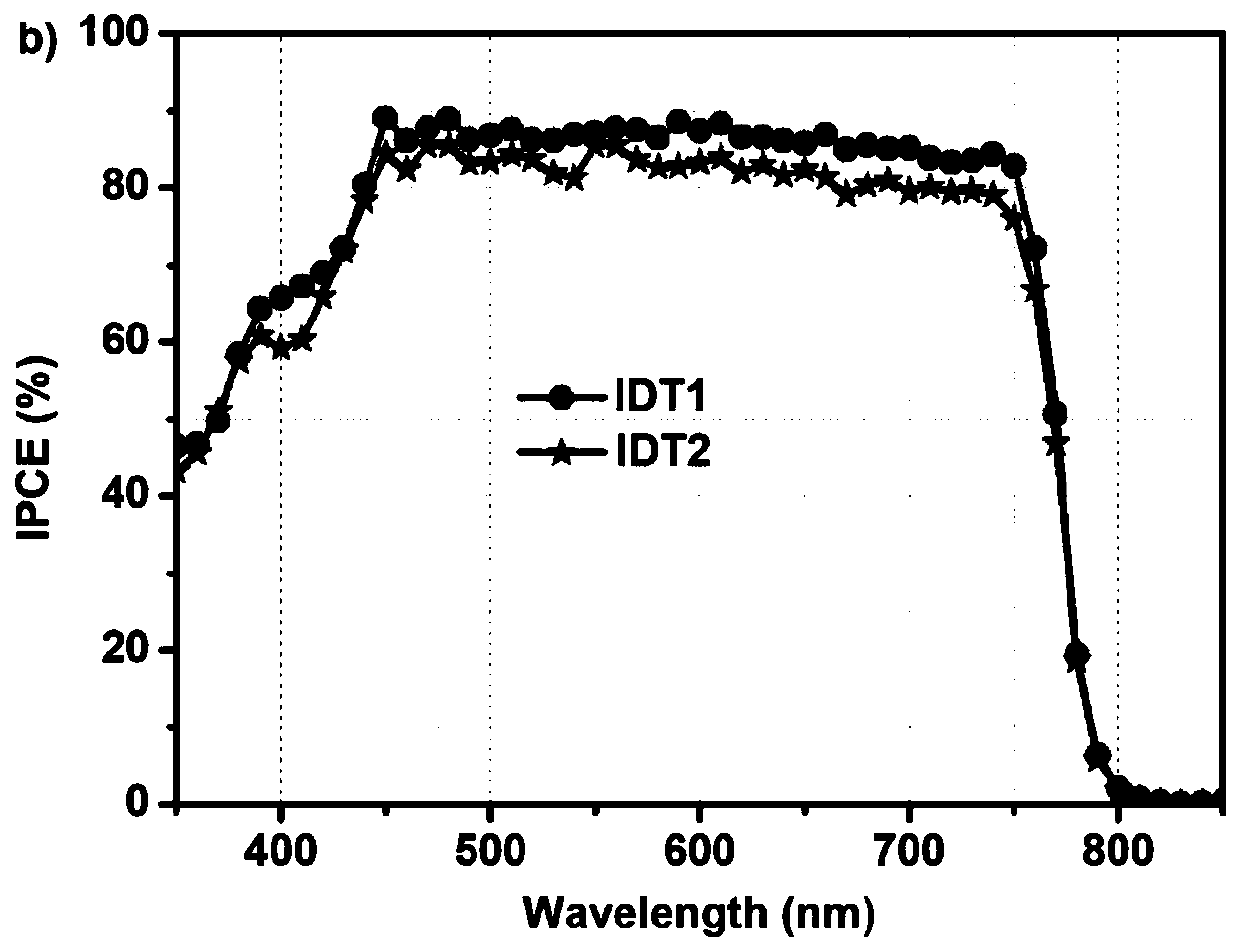
Hole transporting material with indacenodithiophene as core, synthesis method thereof and application thereof
A technology of hole transport material and dithiophene, which is applied in photovoltaic power generation, electrical components, climate sustainability, etc., can solve the problems of complex synthesis and purification steps, increase the cost of battery production, limit practicality, etc., and achieve thermal stability And the effect of good chemical stability, improved photoelectric performance, and adjustable energy level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of hole transport material IDT1:
[0039]
[0040] (i) Add 4,4,9,9-tetrakis(4-n-hexylphenyl)indaeodithiophene derivative (0.725g, 0.8mmol) and solvent tetrahydrofuran THF (30mL) into a dry reaction vessel, Fix the reaction vessel on an iron stand, and stir in an ice-water bath. While stirring, add N-bromosuccinimide (NBS, 0.285g, 1.6mmol) in small amounts, react for 2 hours, and then warm up to room temperature , reacted for 6h, after the reaction was over, added an aqueous solution (100mL) to the reaction solution, extracted three times with dichloromethane (150mL), collected the organic layer, removed the organic solvent under reduced pressure, and separated the resulting solid with a silica gel column. , petroleum ether as the eluent, and vacuum-dried to obtain white solid compound 1 (0.809 g, yield 95.2%). 1 H NMR (400MHz, Chloroform-d) δ7.35(s,2H),7.10(q,J=8.4Hz,16H),7.01(s,2H),2.58(t,J=7.8Hz,8H),1.58 (s,8H),1.40–1.27(m,24H),0.96–0.85(m,12H).
[004...
example 2
[0045] Synthesis of hole transport material IDT2:
[0046]
[0047] (i) Add 4,4,9,9-tetra-n-hexylindaprodithiophene derivatives (0.482g, 0.8mmol) and solvent tetrahydrofuran THF (30mL) into a dry reaction vessel, and fix the reaction vessel on an iron Stand on the stand, and stir in an ice-water bath, while stirring, add N-bromosuccinimide (NBS, 0.285g, 1.6mmol) in small amounts, react for 1.5h, warm up to room temperature, react for 5h, react After the end, add aqueous solution (100mL) to the reaction solution, extract three times with dichloromethane (150mL), collect the organic layer, remove the organic solvent under reduced pressure, separate the obtained solid with a silica gel column, and use petroleum ether as the washing agent. The solvent was removed and dried in vacuo to obtain white solid compound 1 (0.583 g, yield 96.1%). 1 H NMR (400MHz, Chloroform-d) δ7.20(s,2H),6.99(s,2H),1.90(dddd,J=43.9,13.3,11.6,4.8Hz,8H),1.20–1.07(m,24H ),0.81(t,J=6.9Hz,20H).
[0048] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com