Asymmetric fused heterocyclic small molecule electron acceptor material based on dithienopyrrole and application thereof
An electron acceptor material, phenopyrrole technology, which is applied in the field of organic solar cell materials, can solve the problems that there is no asymmetric acceptor material, the research direction of asymmetric small molecule acceptor is not deep enough, etc., and achieves good energy level matching. , the effect of wide light absorption range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
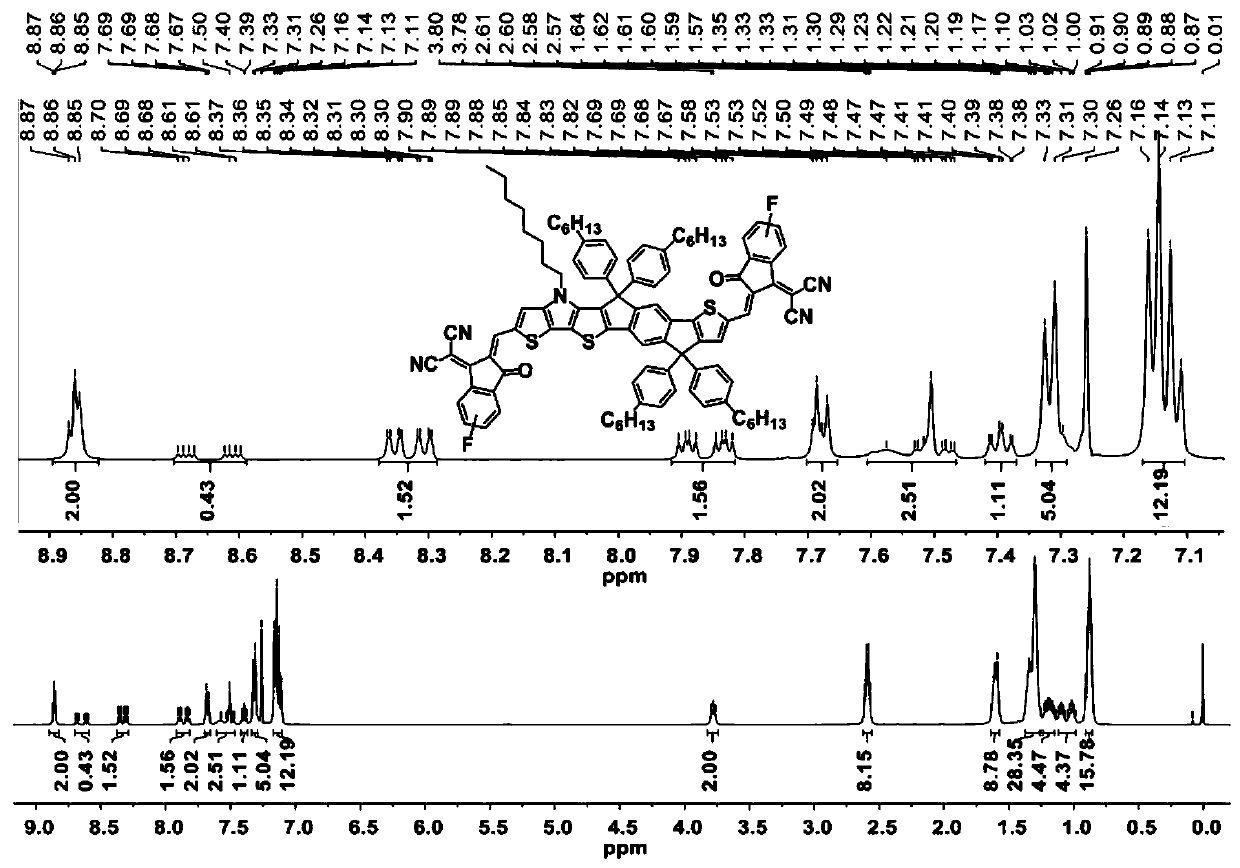
[0053] Example 1 takes the synthesis of IPT-2F as an example. Synthesis of compound 3:
[0054]
[0055] Under nitrogen protection, 2,5-dibromodiethyl terephthalate (compound 1) (3.93g, 10.34mmol), compound 2 (4.00g, 6.89mmol), and dry toluene (100mL) were added to In a 250mL double-necked flask, deoxygenate by bubbling nitrogen for half an hour, then quickly add Pd(PPh 3 ) 4 (159.25mg, 137.81μmol), the reaction solution was heated to 100°C and reacted for 48 hours. After the reaction stopped, the reaction solution was cooled to room temperature, and the toluene solvent was evaporated with a rotary evaporator. Then separated and purified by chromatographic column, using petroleum ether / ethyl acetate as eluent (15:1 by volume ratio), to obtain orange viscous liquid 3 (2.20 g, 54%). 1 H NMR (500MHz, CDCl 3 , δ): 7.95(d, J=1.9Hz, 2H), 7.17(d, J=5.3Hz, 1H), 7.04(s, 1H), 7.00(d, J=5.3Hz, 1H), 4.44(m , 2H), 4.24(m, 2H), 4.18(t, J=7.0Hz, 2H), 1.86(m, 2H), 1.42(t, J=7.1Hz, 3H...
Embodiment 2
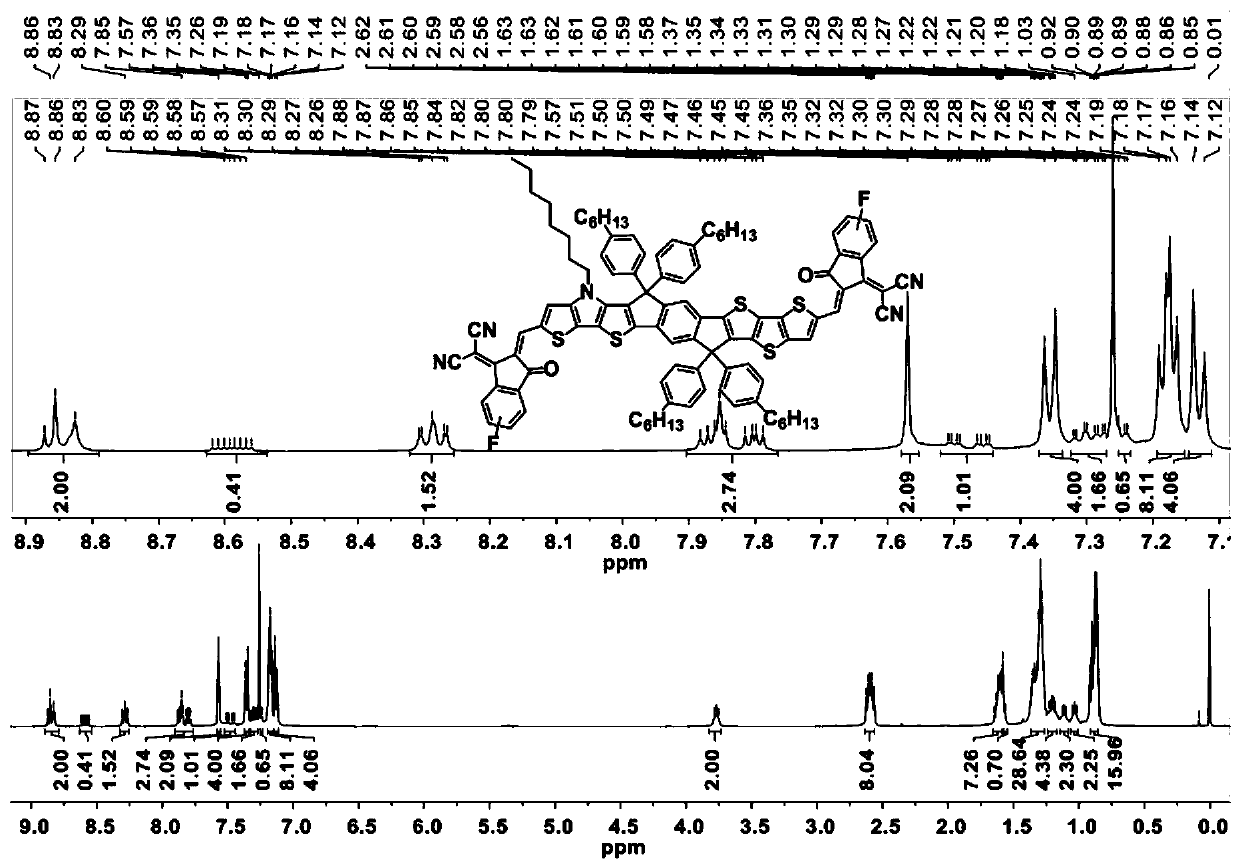
[0069] Example 2 takes the synthesis of IPTT-2F as an example. Synthesis of Compound 8:
[0070]
[0071] Under the protection of nitrogen, sequentially add compound 3 (500 mg, 0.85 mmol), compound 5 (472.45 mg, 1.10 mmol), and dry toluene (12 mL) into a 20 mL reaction flask. Pd(PPh 3 ) 4 (48.92mg, 42.33μmol), the reaction solution was heated to 110°C and reacted for 12 hours. After the reaction stopped, the reaction liquid was cooled to room temperature, and the toluene solvent was evaporated with a rotary evaporator. Then separated and purified by chromatographic column, using petroleum ether / dichloromethane as eluent (volume ratio: 3:2), to obtain orange viscous liquid 8 (450 mg, 81%). 1 H NMR (500MHz, CDCl 3 , δ): 7.95(s, 1H), 7.85(s, 1H), 7.40(d, J=5.2Hz, 1H), 7.33-7.29(m, 2H), 7.18(d, J=5.3Hz, 1H) , 7.10(s, 1H), 7.02(d, J=5.3Hz, 1H), 4.28(m, 4H), 4.20(t, J=7.0Hz, 2H), 1.88(m, 2H), 1.36-1.22( m, 10H), 1.17(m, 6H), 0.87(t, J=6.8Hz, 3H). 13 C NMR (125MHz, CDCl 3...
Embodiment 3
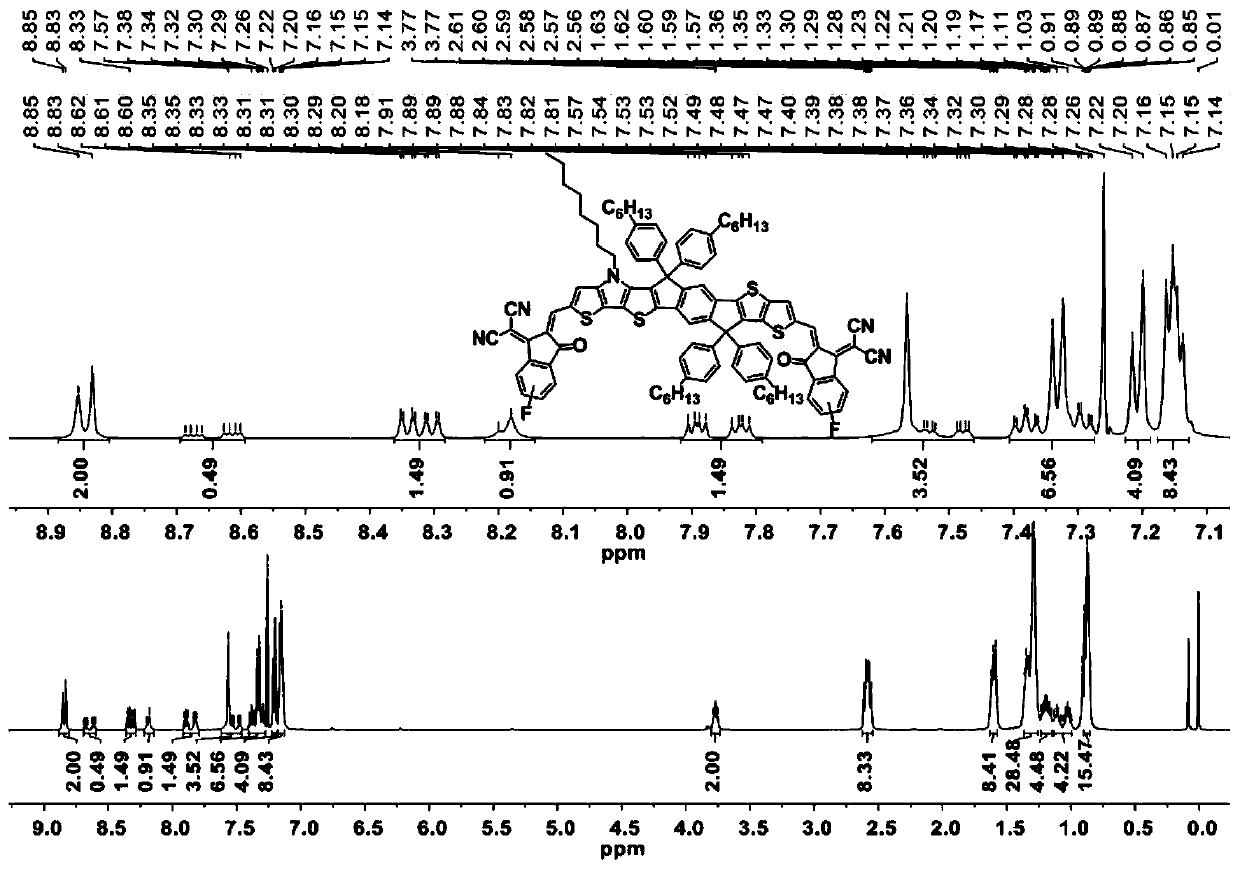
[0082] Example 3 takes the synthesis of IPTTT-2F as an example. Synthesis of Compound 9:
[0083]
[0084] Under the protection of nitrogen, sequentially add compound 3 (500 mg, 0.85 mmol), compound 6 (534.17 mg, 1.10 mmol), and dry toluene (12 mL) into a 20 mL reaction flask. Pd(PPh 3 ) 4 (48.92mg, 42.33μmol), the reaction solution was heated to 110°C and reacted for 12 hours. After the reaction stopped, the reaction liquid was cooled to room temperature, and the toluene solvent was evaporated with a rotary evaporator. Subsequently, it was separated and purified by chromatographic column, using petroleum ether / dichloromethane as eluent (volume ratio 1:1), to obtain yellow solid 9 (500 mg, 84%). 1 H NMR (500MHz, CDCl 3 , δ): 7.95(s, 1H), 7.85(s, 1H), 7.40(d, J=5.2Hz, 1H), 7.33-7.29(m, 2H), 7.18(d, J=5.3Hz, 1H) , 7.10(s, 1H), 7.02(d, J=5.3Hz, 1H), 4.28(m, 4H), 4.20(t, J=7.0Hz, 2H), 1.88(m, 2H), 1.36-1.22( m, 10H), 1.17(m, 6H), 0.87(t, J=6.8Hz, 3H). 13 C NMR (125MHz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com