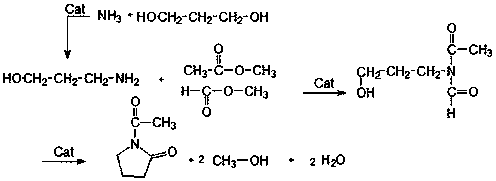
Production method of 1-acetyl-2-pyrrolidone
A technology for pyrrolidone and its production method, which is applied in the production field of 1-acetyl-2-pyrrolidone, can solve the problems of cumbersome operation process, decreased safety factor, high equipment requirements, etc., and achieve prolongation of reaction time, short reaction time and high activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A production method of 1-acetyl-2-pyrrolidone, comprising the following steps:
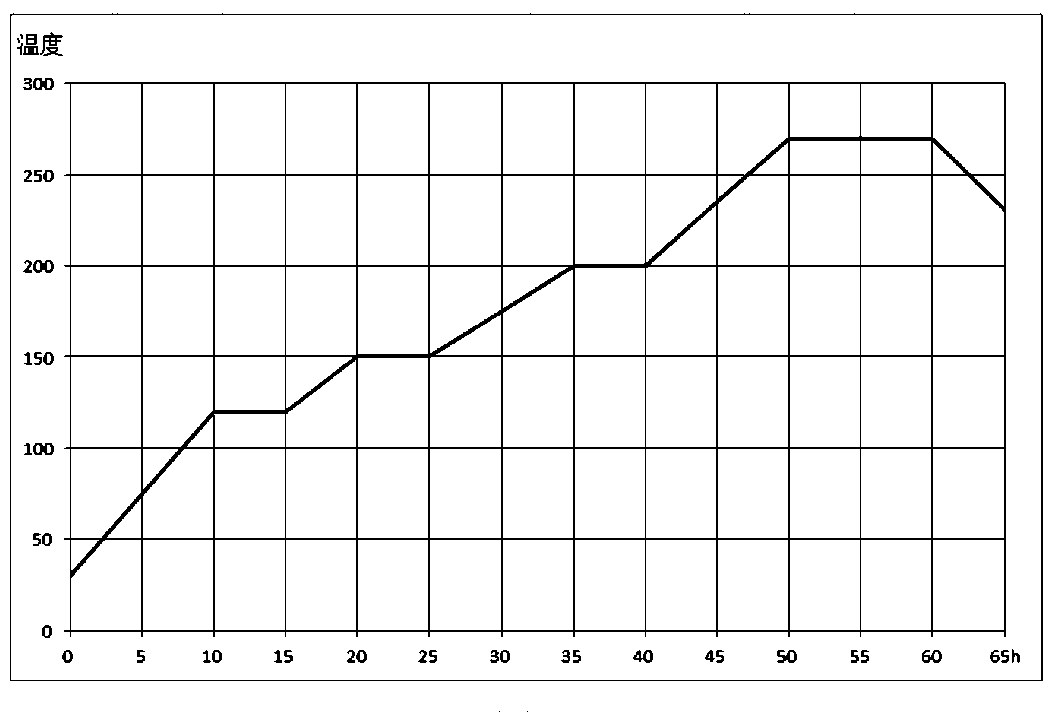
[0034] (1) Dissolve copper nitrate, bismuth nitrate and silver nitrate in deionized water to form a 1.5mol / L solution A, then add polyethylene glycol with a polymerization degree of 2000, stir, and transfer to a microwave hydrothermal parallel synthesizer Under the condition of 80 ℃, react for 6h to obtain the mixed solution B containing Cu / Bi / Ag; 2 o 3 and TiO 2 Use ultrasonic waves to disperse in deionized water to form solution C; use ultrasonic waves to disperse the nano-scale carrier in deionized water to form solution D; mix solutions B, C, and D in parallel, stir for 24 hours, and add a precipitant dropwise to control The pH at the end point is 6.8, let stand, filter, wash, and vacuum-dry the filter cake, then roast at 500°C for 10 hours to obtain the catalyst;
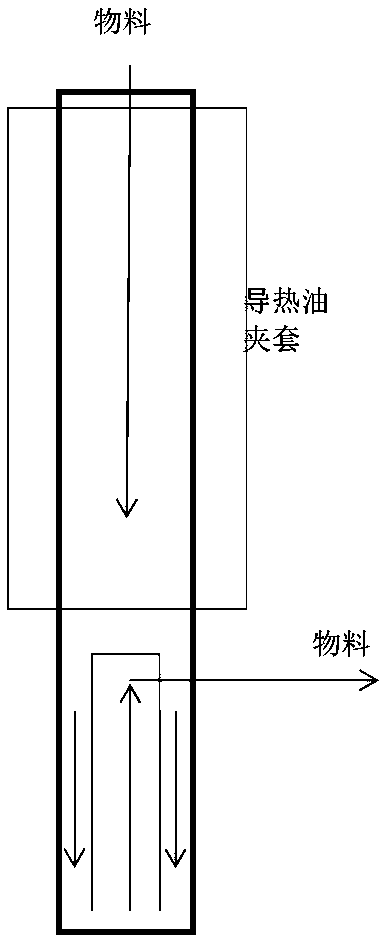
[0035] (2) Pre-pack the catalyst obtained in step (1) in a tubular fixed-bed reactor, use nitrogen to replace the react...
Embodiment 2
[0046] A production method of 1-acetyl-2-pyrrolidone, comprising the following steps:
[0047] (1) Dissolve copper nitrate, bismuth nitrate and silver nitrate in deionized water to prepare 1mol / L solution A, then add polyethylene glycol with a degree of polymerization of 8000, stir, and move to a microwave hydrothermal parallel synthesizer Under the condition of 90°C, react for 5h to obtain a mixed solution B containing Cu / Bi / Ag; 2 o 3 and TiO 2 Use ultrasonic waves to disperse in deionized water to form solution C; use ultrasonic waves to disperse the nano-scale carrier in deionized water to form solution D; mix solutions B, C, and D in parallel, stir for 20 hours, and add a precipitant dropwise to control The end point pH is 6.7, let stand, filter, wash, vacuum-dry the filter cake and roast at 550°C for 8 hours to obtain the catalyst;
[0048] (2) Pre-pack the catalyst obtained in step (1) in a tubular fixed-bed reactor, use nitrogen to replace the reactor, and hydrogen t...
Embodiment 3
[0059] A production method of 1-acetyl-2-pyrrolidone, comprising the following steps:
[0060] (1) Dissolve copper nitrate, bismuth nitrate and silver nitrate in deionized water to make solution A of 1.5mol / L, then add polyethylene glycol with a polymerization degree of 6000, stir, and transfer to microwave hydrothermal parallel synthesizer Under the condition of 80 ℃, react for 8h to obtain the mixed solution B containing Cu / Bi / Ag; 2 o 3 and TiO 2 Use ultrasonic waves to disperse in deionized water to form solution C; use ultrasonic waves to disperse the nano-scale carrier in deionized water to form solution D; mix solutions B, C, and D in parallel, stir for 24 hours, and add a precipitant dropwise to control The pH at the end point is 7.0, let stand, filter, wash, vacuum-dry the filter cake and then roast at 550°C for 10 hours to obtain the catalyst;
[0061] (2) Pre-pack the catalyst obtained in step (1) in a tubular fixed-bed reactor, use nitrogen to replace the reactor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com